 | |
| Names | |
|---|---|
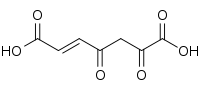
| Preferred IUPAC name
(2E)-4,6-Dioxohept-2-enedioic acid | |
| Other names
(E)-4,6-Dioxohept-2-enedioic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C7H6O6 | |
| Molar mass | 186.119 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3-Fumarylpyruvic acid, or 3-fumarylpyruvate, is a dicarboxylic acid formed from the isomerisation of 3-maleylpyruvate by maleylpyruvate isomerase.[1] It is converted into fumarate and pyruvate by 3-fumarylpyruvate hydrolase.[2]
References
- ↑ Lack L (1961). "Enzymic cis-trans isomerization of maleylpyruvic acid". J. Biol. Chem. 236 (11): 2835–2840. doi:10.1016/S0021-9258(19)76386-8. PMID 14461395.
- ↑ Qu, Y. & Spain, J.C. (2011). "Molecular and biochemical characterization of the 5-nitroanthranilic acid degradation pathway in Bradyrhizobium sp. strain JS329". J. Bacteriol. 193 (12): 3057–3063. doi:10.1128/JB.01188-10. PMC 3133195. PMID 21498645.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.