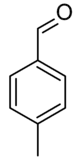
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Methylbenzaldehyde | |||
| Other names
p-Tolualdehyde; p-Tolylaldehyde | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.952 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H8O | |||
| Molar mass | 120.14852 | ||
| Appearance | colorless liquid | ||
| Density | 1.019 g/mL (25 °C) | ||
| Melting point | −6.00 °C (21.20 °F; 267.15 K) | ||
| Boiling point | 204 to 205 °C (399 to 401 °F; 477 to 478 K) | ||
Refractive index (nD) |
1.545 (20 °C) | ||
| Hazards | |||
| Safety data sheet (SDS) | |||
| Related compounds | |||
Related compounds |
Benzaldehyde | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
4-Methylbenzaldehyde is the aromatic aldehyde with the formula CH3C6H4CHO. It is a colorless liquid. Commercially available, it may be prepared from the Friedel-Crafts formylation of toluene with carbon monoxide and hydrogen chloride under Gattermann-Koch conditions.[1] 4-Methylbenzaldehyde has a cherry-like scent similar to benzaldehyde.
References
- ↑ Coleman, G. H.; Craig, David (1932). "p-Tolualdehyde". Org. Synth. 12: 80. doi:10.15227/orgsyn.012.0080.; Coll. Vol., vol. 2, 1943, p. 583
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.