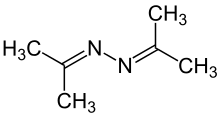
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Di(propan-2-ylidene)hydrazine | |||
| Systematic IUPAC name
Acetone azine | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 4-01-00-03207 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.009 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H12N2 | |||
| Molar mass | 112.176 g·mol−1 | ||
| Appearance | Pale-yellow liquid | ||
| Density | 0.842 g cm−3 | ||
| Melting point | −125 °C (−193 °F; 148 K) | ||
| Boiling point | 133 °C (271 °F; 406 K) | ||
Refractive index (nD) |
1.454 | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H226, H302, H311, H315, H319, H335, H350 | |||
| P201, P261, P280, P305+P351+P338, P308+P313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 31 °C (88 °F; 304 K) | ||
| Related compounds | |||
Related compounds |
Hydrazine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Acetone azine is the simplest ketazine. It is an intermediate in some hydrazine manufacturing processes.
Synthesis
Acetone azine can be prepared from acetone and hydrazine:[3]
- 2 (CH3)2CO + N2H4 → 2 H2O + [(CH3)2C=N]2
It can also be produced from acetone (2 eq.), ammonia (2 eq.) and hydrogen peroxide (1 eq.).[4] The first step is the formation of acetone imine, Me2C=NH; this is then oxidized by hydrogen peroxide through a complex mechanism to give 3,3-dimethyloxaziridine, which reacts with a further molecule of ammonia to produce acetone hydrazone. The hydrazone then condenses with a further molecule of acetone to produce the azine. The acetone azine product is distilled out of the reaction mixture as a 1:6 azeotrope with water.[5]
Reactions
Acetone azine can be used to prepare acetone hydrazone[3] and 2-diazopropane.[6]
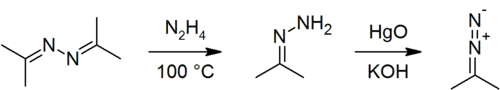
Hydrazine can be produced through acid-catalysed hydrolysis of acetone azine:[7]
- 2 H2O + [(CH3)2C=N]2 → 2 (CH3)2CO + N2H4
References
- ↑ "Acetone azine MSDS (Santa Cruz Biotechnology)" (PDF).
- ↑ "Acetone azine MSDS (Sigma Aldrich)".
- 1 2 Day, A. C.; Whiting, M. C. (1970). "Acetone Hydrazone". Organic Syntheses: 3.; Collective Volume, vol. 6, 1988, p. 10
- ↑ US 3972878, Schirmann, Jean-Pierre; Combroux, Jean & Delavarenne, Serge Yvon, "Method for preparing azines and hydrazones", issued 1976-08-03, assigned to Produits Chimiques Ugine Kuhlmann.US 3978049, Schirmann, Jean-Pierre; Tellier, Pierre & Mathais, Henri et al., "Process for the preparation of hydrazine compounds", issued 1976-08-31, assigned to Produits Chimiques Ugine Kuhlmann
- ↑ US 4724133, Schirmann, Jean-Pierre; Combroux, Jean & Delavarenne, Serge Y., "Preparation of a concentrated aqueous solution of hydrazine hydrate", issued 1988-02-09, assigned to Atochem
- ↑ Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. (1970). "2-Diazopropane". Organic Syntheses: 27.; Collective Volume, vol. 6, 1988, p. 392
- ↑ Gilbert, E. C. (1929), "Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone", J. Am. Chem. Soc., 51 (11): 3394–3409, doi:10.1021/ja01386a032.