 | |
 | |
| Names | |
|---|---|
| IUPAC name
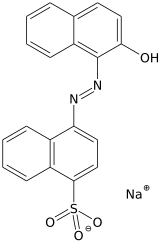
Sodium 4-(2-hydroxy-1-naphthalenylazo)-naphthalenesulfonate | |
| Other names
Fast Red A 2-Naphthol Red | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.238 |
| EC Number |
|
| MeSH | Fast+red+S |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H13N2NaO4S | |
| Molar mass | 400.38 g·mol−1 |
| Appearance | Vivid, dark red, opaque, vitreous solid |
| Melting point | 280 °C (536 °F; 553 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
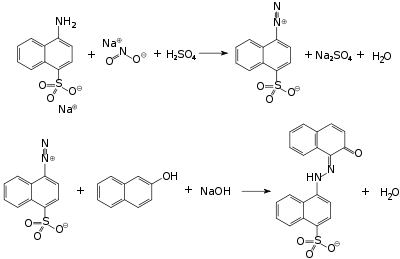
Acid red 88 is an azo dye. Due to its intense colour, solid samples appear almost black. It is used to dye cotton textiles red.[1] A closely related acid dye is Acid Red 13.
Preparation and use
It can be obtained by azo coupling of naphthionic acid and 2-naphthol. Instead of crystallising, it vitrifies when cooled or salted out of the solution.
This compound is used in the textile industry as a dye.[2] It can also be used for research in photocatalysis (as degradation object).[3]
References
- ↑ Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 3527306730.
- ↑ Song, Ya-Li; Li, Ji-Tai; Chen, Hua (2009). "Degradation of C.I. Acid Red 88 aqueous solution by combination of Fenton's reagent and ultrasound irradiation". J. Chem. Technol. Biotechnol. 84 (4): 578–583. doi:10.1002/jctb.2083.
- ↑ "Acid red 88 | CAS 1658-56-6 | Santa Cruz Biotech". Archived from the original on 2015-07-12. Retrieved 2011-04-24.
External links
- echo Chemical Database: 1-Naphthalenesulfonic acid, 4-((2-hydroxy-1-naphthalenyl)azo)-, monosodium salt (EnvironmentalChemistry.com)- This page contains information on the chemical 1-Naphthalenesulfonic acid, 4-((2-hydroxy-1-naphthalenyl)azo)-, monosodium salt including: 72 synonyms/identifiers.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.