 | |
| Names | |
|---|---|
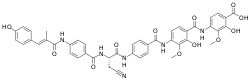
| IUPAC name
4-[[4-[[4-[[(2S)-3-cyano-2-[[4-[[(E)-3-(4-hydroxyphenyl)-2-methylprop-2-enoyl]amino]benzoyl]amino]propanoyl]amino]benzoyl]amino]-2-hydroxy-3-methoxybenzoyl]amino]-2-hydroxy-3-methoxybenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C44H38N6O12 | |
| Molar mass | 842.818 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Albicidin is an antibiotic and phytotoxic molecule produced by the bacterium Xanthomonas albilineans which infects sugarcane causing leaf scald.[1]
As a phytotoxin, it acts by inhibiting the differentiation of chloroplasts.[2] It accomplishes this by inhibiting DNA gyrase, and thereby preventing the replication of chloroplast DNA.[3] As such it plays a major role in leaf scald disease.
As a DNA gyrase inhibitor, albicindin also has potential therapeutic use as an antibiotic.[4] Its antibiotic properties were discovered in the early 1980s, when the molecule was isolated and purified from cultures of Xanthomonas albilineans.[5] However, the precise structure of the molecule was only identified in 2015.[6] A laboratory synthesis of albicidin has been developed,[1] and research is currently focused on the design and evaluation of synthetic derivatives of albicidin with improved properties.[7][8][9]
References
- 1 2 Kretz, Julian; Kerwat, Dennis; Schubert, Vivien; Grätz, Stefan; Pesic, Alexander; Semsary, Siamak; Cociancich, Stéphane; Royer, Monique; Süssmuth, Roderich D. (2015). "Total Synthesis of Albicidin: A Lead Structure from Xanthomonas albilineansfor Potent Antibacterial Gyrase Inhibitors". Angewandte Chemie International Edition. 54 (6): 1969–1973. doi:10.1002/anie.201409584. PMID 25504839.
- ↑ Pieretti, Isabelle; Pesic, Alexander; Petras, Daniel; Royer, Monique; Süssmuth, Roderich D.; Cociancich, Stéphane (2015). "What makes Xanthomonas albilineans unique amongst xanthomonads?". Frontiers in Plant Science. 6: 289. doi:10.3389/fpls.2015.00289. PMC 4408752. PMID 25964795.
- ↑ Hashimi, Saeed M.; Wall, Melisa K.; Smith, Andrew B.; Maxwell, Anthony; Birch, Robert G. (2007). "The Phytotoxin Albicidin is a Novel Inhibitor of DNA Gyrase". Antimicrobial Agents and Chemotherapy. 51 (1): 181–187. doi:10.1128/AAC.00918-06. PMC 1797663. PMID 17074789.
- ↑ Hashimi, Saeed Mujahid (2019). "Albicidin, a potent DNA gyrase inhibitor with clinical potential". The Journal of Antibiotics. 72 (11): 785–792. doi:10.1038/s41429-019-0228-2. PMID 31451755. S2CID 201644516.
- ↑ Birch, R. G.; Patil, S. S. (1985). "Preliminary Characterization of an Antibiotic Produced by Xanthomonas albilineans Which Inhibits DNA Synthesis in Escherichia coli". Microbiology. 131 (5): 1069–1075. doi:10.1099/00221287-131-5-1069. PMID 2410547.
- ↑ Cociancich, Stéphane; Pesic, Alexander; Petras, Daniel; Uhlmann, Stefanie; Kretz, Julian; Schubert, Vivien; Vieweg, Laura; Duplan, Sandrine; Marguerettaz, Mélanie; Noëll, Julie; Pieretti, Isabelle; Hügelland, Manuela; Kemper, Sebastian; Mainz, Andi; Rott, Philippe; Royer, Monique; Süssmuth, Roderich D. (2015). "The gyrase inhibitor albicidin consists of p-aminobenzoic acids and cyanoalanine". Nature Chemical Biology. 11 (3): 195–197. doi:10.1038/nchembio.1734. PMID 25599532.
- ↑ Kerwat, Dennis; Grätz, Stefan; Kretz, Julian; Seidel, Maria; Kunert, Maria; Weston, John B.; Süssmuth, Roderich D. (2016). "Synthesis of Albicidin Derivatives: Assessing the Role of N-terminal Acylation on the Antibacterial Activity". ChemMedChem. 11 (17): 1899–1903. doi:10.1002/cmdc.201600231. PMID 27439374. S2CID 5009104.
- ↑ Grätz, Stefan; Kerwat, Dennis; Kretz, Julian; Von Eckardstein, Leonard; Semsary, Siamak; Seidel, Maria; Kunert, Maria; Weston, John B.; Süssmuth, R. D. (2016). "Synthesis and Antimicrobial Activity of Albicidin Derivatives with Variations of the Central Cyanoalanine Building Block". ChemMedChem. 11 (14): 1499–1502. doi:10.1002/cmdc.201600163. PMID 27245621. S2CID 205649206.
- ↑ "Sweet salvation -- how a sugar cane pathogen is gearing up a new era of antibiotic discovery". Science Daily. January 23, 2023.