Ammonia production takes place worldwide, mostly in large-scale manufacturing plants that produce 183 million metric tonnes[1] of ammonia (2021) annually.[2][3] Leading producers are China (31.9%), Russia (8.7%), India (7.5%), and the United States (7.1%). 80% or more of ammonia is used as fertilizer. Ammonia is also used for the production of plastics, fibres, explosives, nitric acid (via the Ostwald process), and intermediates for dyes and pharmaceuticals. The industry contributes 1% to 2% of global CO
2.[4] Between 18-20 Mt of the gas is transported globally each year.[5]
History
Dry distillation
Before the start of World War I, most ammonia was obtained by the dry distillation of nitrogenous vegetable and animal products; by the reduction of nitrous acid and nitrites with hydrogen; and also by the decomposition of ammonium salts by alkaline hydroxides or by quicklime, the salt most generally used being the chloride (sal-ammoniac).

Frank–Caro process
Adolph Frank and Nikodem Caro found that Nitrogen could be fixed by using the same calcium carbide produced to make acetylene to form calcium-cyanamide, which could then be divided with water to form ammonia. The method was developed between 1895 and 1899.
Birkeland–Eyde process
While not strictly speaking a method of producing ammonia, nitrogen can be fixed by passing it (with oxygen) through an electric spark.
Nitrides
Heating metals such as magnesium in an atmosphere of pure nitrogen produces the nitride, which when combined with water produce the metal hydroxide and ammonia.
Haber-Bosch process

The Haber process,[7] also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia.[8][9] The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures[10] and higher pressures.[11] It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.
The primary reaction is:
Sustainable production

Ammonia production depends on plentiful supplies of energy. Sustainable production is possible by using non-polluting methane pyrolysis or generating hydrogen by water electrolysis with renewable energy sources.[15] Thyssenkrupp Uhde Chlorine Engineers expanded its annual production capacity for alkaline water electrolysis to 1 gigawatt of electrolyzer capacity for this purpose.[16]
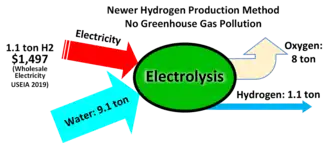
In a hydrogen economy some hydrogen production could be diverted to feedstock use. For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess power from its hydroelectric plants, primarily for fertilizer.[17] The Vemork hydroelectric plant in Norway used its surplus electricity output to generate renewable nitric acid from 1911 to 1971,[18] requiring 15 mWh/ton of nitric acid. The same reaction is carried out by lightning, providing a natural source of soluble nitrates.[19] Natural gas remains the lowest cost method.
Wastewater is often high in ammonia. Because discharging ammonia-laden water into the environment damages marine life, nitrification is often necessary to remove the ammonia.[20] This may become a potentially sustainable source of ammonia given its abundance.[21] Alternatively, ammonia from wastewater can be sent into an ammonia electrolyzer (ammonia electrolysis) operating with renewable energy sources to produce hydrogen and clean water.[22] Ammonia electrolysis may require much less thermodynamic energy than water electrolysis (only 0.06 V in alkaline media).[23]
Another option for recovering ammonia from wastewater is to use the mechanics of the ammonia-water thermal absorption cycle.[24][25] Ammonia can thus be recovered either as a liquid or as ammonium hydroxide. The advantage of the former is that it is much easier to handle and transport, whereas the latter has commercial value at concentrations of 30 percent in solution.
Coal

Making ammonia from coal is mainly practised in China, where it is the main source.[6] Oxygen from the air separation module is fed to the gasifier to convert coal into synthesis gas (H2, CO, CO2) and CH4. Most gasifiers are based on fluidized beds that operate above atmospheric pressure and have the ability to utilize different coal feeds.
Production plants
The American Oil Co in the mid-1960s positioned a single-converter ammonia plant engineered by M. W. Kellogg at Texas City, Texas, with a capacity of 544 m.t./day. It used a single-train design that received the “Kirkpatrick Chemical Engineering Achievement Award” in 1967. The plant used a four-case centrifugal compressor to compress the syngas to a pressure of 152 bar Final compression to an operating pressure of 324 bar occurred in a reciprocating compressor. Centrifugal compressors for the synthesis loop and refrigeration services provided significant cost reductions.
Almost every plant built between 1964 and 1992 had large single-train designs with syngas manufacturing at 25–35 bar and ammonia synthesis at 150–200 bar. Braun Purifier process plants utilized a primary or tubular reformer with a low outlet temperature and high methane leakage to reduce the size and cost of the reformer. Air was added to the secondary reformer to reduce the methane content of the primary reformer exit stream to 1–2%. Excess nitrogen and other impurities were erased downstream of the methanator. Because the syngas was essentially free of impurities, two axial-flow ammonia converters were used. In early 2000 Uhde developed a process that enabled plant capacities of 3300 mtpd and more. The key innovation was a single-flow synthesis loop at medium pressure in series with a conventional high-pressure synthesis loop.[26]
Small-scale onsite plants
In April 2017, Japanese company Tsubame BHB implemened a method of ammonia synthesis that could allow economic production at scales 1-2 orders of magnitude below than ordinary plants with utilizing electrochemical catalyst.[27][28]
Byproducts and shortages due to shutdowns
One of the main industrial byproducts of ammonia production is CO2. In 2018, high oil prices resulted in an extended summer shutdown of European ammonia factories causing a commercial CO2 shortage, thus limiting production of CO2-based products such as beer and soft drinks.[29] This situation repeated in September 2021 due to a 250-400% increase in the wholesale price of natural gas over the course of the year.[30][31]
See also
References
- ↑ Congressional Research Service. (7 December 2022). "Ammonia’s Potential Role in a Low-Carbon Economy". CRP website Retrieved 24 September 2023.
- ↑ "Global ammonia annual production capacity".
- ↑ "Mitsubishi Heavy Industries BrandVoice: Scaling Ammonia Production for the World's Food Supply". Forbes.
- ↑ Koop, Fermin (2023-01-13). "Green ammonia (and fertilizer) may finally be in sight -- and it would be huge". ZME Science. Retrieved 2023-03-21.
- ↑ Congressional Research Service. (7 December 2022). "Ammonia’s Potential Role in a Low-Carbon Economy". CRP website Retrieved 24 September 2023.
- 1 2 "Introduction to Ammonia Production". www.aiche.org. 2016-09-08. Retrieved 2021-08-19.
- ↑ Habers process chemistry. India: Arihant publications. 2018. p. 264. ISBN 978-93-131-6303-9.
- ↑ Appl, M. (1982). "The Haber–Bosch Process and the Development of Chemical Engineering". A Century of Chemical Engineering. New York: Plenum Press. pp. 29–54. ISBN 978-0-306-40895-3.
- ↑ Appl, Max (2006). "Ammonia". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_143.pub2. ISBN 978-3527306732.
- ↑ Clark 2013, "The forward reaction (the production of ammonia) is exothermic. According to Le Chatelier's Principle, this will be favoured at a lower temperature. The system will respond by moving the position of equilibrium to counteract this – in other words by producing more heat. To obtain as much ammonia as possible in the equilibrium mixture, as low a temperature as possible is needed".
- ↑ Clark 2013, "Notice that there are 4 molecules on the left-hand side of the equation, but only 2 on the right. According to Le Chatelier's Principle, by increasing the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again. To get as much ammonia as possible in the equilibrium mixture, as high a pressure as possible is needed. 200 atmospheres is a high pressure, but not amazingly high".
- ↑ Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (1st ed.). Cambridge, MA: MIT. ISBN 978-0-262-69313-4.
- ↑ Hager, Thomas (2008). The Alchemy of Air: A Jewish genius, a doomed tycoon, and the scientific discovery that fed the world but fueled the rise of Hitler (1st ed.). New York, New York: Harmony Books. ISBN 978-0-307-35178-4.
- ↑ Sittig, Marshall (1979). Fertilizer Industry: Processes, Pollution Control, and Energy Conservation. Park Ridge, New Jersey: Noyes Data Corp. ISBN 978-0-8155-0734-5.
- ↑ Lumbers, Brock (2022). "Mathematical modelling and simulation of the thermo-catalytic decomposition of methane for economically improved hydrogen production". International Journal of Hydrogen Energy. 47 (7): 4265–4283. doi:10.1016/j.ijhydene.2021.11.057. S2CID 244814932. Retrieved 16 March 2022.
- ↑ "Water Electrolysis > Products > Home". Uhde Chlorine Engineers. Retrieved 2021-12-08.
- ↑ "Iceland launches energy revolution". BBC News. 2001-12-24. Archived from the original on 7 April 2008. Retrieved 2008-03-23.
- ↑ Bradley, David (2004-02-06). "A Great Potential: The Great Lakes as a Regional Renewable Energy Source" (PDF). Archived from the original (PDF) on 29 October 2008. Retrieved 2008-10-04.
- ↑ Karl Fisher; William E. Newton (2002). G. J. Leigh (ed.). Nitrogen fixation at the millennium. Elsevier. pp. 2–3. ISBN 978-0-444-50965-9.
- ↑ "StackPath". www.waterworld.com.
- ↑ Huang, Jianyin; Kankanamge, Nadeeka Rathnayake; Chow, Christopher; Welsh, David T.; Li, Tianling; Teasdale, Peter R. (January 2018). "Removing ammonium from water and wastewater using cost-effective adsorbents: A review". Journal of Environmental Sciences. 63: 174–197. doi:10.1016/j.jes.2017.09.009. PMID 29406102.
- ↑ Muthuvel, Madhivanan; Botte, Gerardine G (2009). "Trends in Ammonia Electrolysis". Modern Aspects of Electrochemistry, No. 45. Modern Aspects of Electrochemistry. Vol. 45. pp. 207–245. doi:10.1007/978-1-4419-0655-7_4. ISBN 978-1-4419-0654-0.
- ↑ Gwak, Jieun; Choun, Myounghoon; Lee, Jaeyoung (February 2016). "Alkaline Ammonia Electrolysis on Electrodeposited Platinum for Controllable Hydrogen Production". ChemSusChem. 9 (4): 403–408. doi:10.1002/cssc.201501046. PMID 26530809.
- ↑ Lin, P.; Wang, R.Z.; Xia, Z.Z.; Ma, Q. (June 2011). "Ammonia–water absorption cycle: a prospective way to transport low-grade heat energy over long distance". International Journal of Low-Carbon Technologies. 6 (2): 125–133. doi:10.1093/ijlct/ctq053.
- ↑ Shokati, Naser; Khanahmadzadeh, Salah (August 2018). "The effect of different combinations of ammonia-water Rankine and absorption refrigeration cycles on the exergoeconomic performance of the cogeneration cycle". Applied Thermal Engineering. 141: 1141–1160. doi:10.1016/j.applthermaleng.2018.06.052. S2CID 115749773.
- ↑ "Das Zweidruckverfahren von Uhde - Düngemittelanlagen". Industrial Solutions (in German). Retrieved 2021-12-08.
- ↑ "Ajinomoto Co., Inc., UMI, and Tokyo Institute of Technology Professors Establish New Company to implement the World's First On Site Production of Ammonia". Ajinomoto. 27 April 2017. Retrieved 22 November 2021.
- ↑ "Technology / Business Introduction". Tsubame BHB. 27 April 2017. Retrieved 22 November 2021.
- ↑ "This is exactly why we're running out of CO2 for beer and meat production". iNews. 2018-06-28.
- ↑ "Why is there a CO2 shortage and how will it hit food supplies?". BBC News. 2021-09-20. Retrieved 2021-09-21.
- ↑ "Gas crisis: No chance lights will go out, says government". BBC News. 2021-09-20. Retrieved 2021-09-21.
Works cited
- Clark, Jim (April 2013) [2002]. "The Haber Process". Retrieved 15 December 2018.
External links
- Today's Hydrogen Production Industry
- Energy Use and Energy Intensity of the U.S. Chemical Industry Archived 2018-09-30 at the Wayback Machine, Report LBNL-44314, Lawrence Berkeley National Laboratory (Scroll down to page 39 of 40 PDF pages for a list of the ammonia plants in the United States)
- Ammonia: The Next Step includes a detailed process flow diagram.
- Ammonia production process plant flow sheet in brief with three controls.