 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.018 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1548 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8ClN | |
| Molar mass | 129.59 g·mol−1 |
| Appearance | white solid |
| Density | 1.68 g/cm3 |
| Melting point | 196 °C (385 °F; 469 K) |
| Boiling point | 245 °C (473 °F; 518 K) |
| 1070 g/l | |
| Hazards | |
| GHS labelling:[1] | |
     | |
| Danger | |
| H301, H311, H317, H318, H331, H341, H351, H372, H400 | |
| P203, P260, P261, P264, P264+P265, P270, P271, P272, P273, P280, P281, P301+P316, P302+P352, P304+P340, P305+P354+P338, P316, P317, P318, P319, P321, P330, P333+P313, P361+P364, P362+P364, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
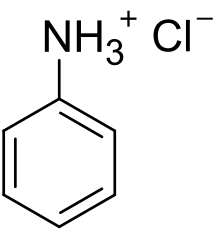
Anilinium chloride is the organic compound with the formula C6H5NH+3Cl−. A white solid, it is the chloride salt of anilinium, which is the conjugate acid of aniline, C6H5NH2. Anilinium chloride is produced by treatment of aniline with hydrochloric acid. The cation consists of a phenyl ring attached to a tetrahedral ammonium center. The C-N bond elongates from 1.41 Å in aniline to 1.474 Å in anilinium.[2]
References
- ↑ "Aniline hydrochloride". pubchem.ncbi.nlm.nih.gov. Retrieved 31 August 2023.
- ↑ Anderson, Kirsty M.; Goeta, Andres E.; Hancock, Kirsty S. B.; Steed, Jonathan W. (2006). "Unusual variations in the incidence of Z? > 1 in oxo-anion structures". Chemical Communications (20): 2138–2140. doi:10.1039/b602492k. PMID 16703133.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
