| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ardisiaquinones are a group of closely related chemical compounds found in plants in the genus Ardisia. The first examples, ardisiaquinones A-C, were isolated in 1968 from Ardisia sieboldii.[1] In 1995, ardisiaquinones D, E, and F were discovered, also from Ardisia sieboldii.[2] In 2001, ardisiaquinones G, H and I were isolated from Ardisia teysmanniana.[3]
Chemically, the ardisiaquinones consist of two variably-substituted 1,4-benzoquinone units connected by a long alkyl or alkenyl chain.[1]
Research
Ardisiaquinones are of research interest because they possess 5-lipoxygenase (5-LOX) inhibitor activity and 5-LOX has clinical relevance in inflammation.[4] For example, ardisiaquinone A protects against liver injury in an animal model of ischemia-reperfusion injury.[5] Likewise, ardisiaquinone G has also shown 5-LOX inhibition.[4] Ardisiaquinone A has also been shown to have an antiallergic effect in an animal model.[6] Other ardisiaquinones have shown antiproliferative and antimicrobial effects in vitro.[7]
Laboratory syntheses of ardisiaquinones A and B have been reported.[8][9]
Chemical structures
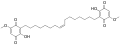 Ardisiaquinone A
Ardisiaquinone A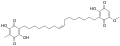 Ardisiaquinone B
Ardisiaquinone B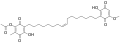 Ardisiaquinone C
Ardisiaquinone C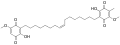 Ardisiaquinone D
Ardisiaquinone D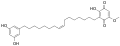 Ardisiaquinone E
Ardisiaquinone E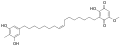 Ardisiaquinone F
Ardisiaquinone F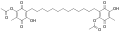 Ardisiaquinone G
Ardisiaquinone G
References
- 1 2 Ogawa, H.; Sakaki, S.; Yoshihira, K.; Natori, S. (1968). "The structures of ardisiaquinones A, B and C, bis(benzoquinonyl)-olefine derivatives from miquel". Tetrahedron Letters. 9 (11): 1387–1392. doi:10.1016/S0040-4039(01)98959-2.
- ↑ Fukuyama, Yoshiyasu; Kiriyama, Yuuko; Kodama, Mitsuaki; Iwaki, Hideyuki; Hosozawa, Shigeki; Aki, Shinji; Matsui, Kuniaki (1995). "Naturally Occurring 5-Lipoxygenase Inhibitors. VI. Structures of Ardisiaquinones D, e, and F from Ardisia sieboldii". Chemical and Pharmaceutical Bulletin. 43 (8): 1391–1394. doi:10.1248/cpb.43.1391. PMID 7553985.
- ↑ Yang, Lay-Kien; Khoo-Beattie, Corinne; Goh, Kay-Lin; Chng, Bee-Lee; Yoganathan, K.; Lai, Yee-Hing; Butler, Mark S. (2001). "Ardisiaquinones from Ardisia teysmanniana". Phytochemistry. 58 (8): 1235–1238. doi:10.1016/s0031-9422(01)00317-x. PMID 11738414.
- 1 2 Narayanaswamy, Radhakrishnan; Veeraragavan, Vijayakumar (2020). "Natural products as antiinflammatory agents". Bioactive Natural Products. Studies in Natural Products Chemistry. Vol. 67. pp. 269–306. doi:10.1016/B978-0-12-819483-6.00008-4. ISBN 9780128194836. S2CID 224940163.
- ↑ Matsui, Nobuaki; Fukuishi, Nobuyuki; Fukuyama, Yoshiyasu; Yasui, Yumiko; Akagi, Masaaki (2005). "Protective Effect of the 5-Lipoxygenase Inhibitor Ardisiaquinone a on Hepatic Ischemia-Reperfusion Injury in Rats". Planta Medica. 71 (8): 717–720. doi:10.1055/s-2005-871252. PMID 16142634.
- ↑ Fukuishi, N.; Takada, T.; Fukuyama, Y.; Akagi, M. (2001). "Antiallergic effect of ardisiaquinone A, a potent 5-lipoxygenase inhibitor". Phytomedicine. 8 (6): 460–464. doi:10.1078/s0944-7113(04)70065-3. PMID 11824521.
- ↑ Paul, Dzoyem J.; Laure, Ndontsa B.; Guru, Santosh K.; Khan, Inshad A.; Ajit, Saxena K.; Vishwakarma, Ram A.; Pierre, Tane (2014). "Antiproliferative and antimicrobial activities of alkylbenzoquinone derivatives from Ardisia kivuensis". Pharmaceutical Biology. 52 (3): 392–397. doi:10.3109/13880209.2013.837076. PMID 24192208. S2CID 35830795.
- ↑ Fukuyama, Yoshiyasu; Kiriyama, Yuuko; Kodama, Mitsuaki; Iwaki, Hideyuki; Hosozawa, Shigeki; Aki, Shinji; Matsui, Kuniaki (1994). "Total Synthesis of Ardisiaquinone A, a Potent 5-Lipoxygenase Inhibitor, Isolated from Ardisia sieboldii, and Degree of 5-Lipoxygenase Inhibitory Activity of Its Derivatives". Chemical and Pharmaceutical Bulletin. 42 (10): 2211–2213. doi:10.1248/cpb.42.2211. PMID 7805143.
- ↑ Fukuyama, Yoshiyasu; Kiriyama, Yuuko; Okino, Junko; Kodama, Mitsuaki; Iwaki, Hideyuki; Hosozawa, Sigeki; Matsui, Kuniaki (1993). "Naturally Occurring 5-Lipoxygenase Inhibitor. II. Structures and Syntheses of Ardisianones a and B, and Maesanin, Alkenyl-1,4-benzoquinones from the Rhizome of Ardisia japonica". Chemical and Pharmaceutical Bulletin. 41 (3): 561–565. doi:10.1248/cpb.41.561. PMID 8477510.