| Ascosphaera aggregata | |
|---|---|
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Subdivision: | |
| Class: | |
| Order: | |
| Genus: | |
| Species: | Ascosphaera aggregata |
| Binomial name | |
| Ascosphaera aggregata Skou, JP (1975)[1] | |
Ascosphaera aggregata is a species of fungus.
History and taxonomy
Ascosphaera aggregata (A. aggregata), discovered in 1975 by Jens-Peder Skou[1] is a fungus that is related to Ascosphaera apis.[2]
Habitat and ecology
A. aggregata is an obligate parasite[3] that causes chalkbrood in bees,[4] symptom manifestations differ depending on age of the larva.[5] It primarily infects alfalfa leafcutting bees, Megachile rotundata.[2][6][7][3][8] Megachile rotundata infected with A. aggregata have been detected in the United States, Canada,[9] and South America.[5] Other bee species that A. aggregata has been seen to infect include the red mason bee (Osmia rufa[8]), the patchwork leafcutter bee (Megachile centuncularis[8]), Megachile pugnata and Megachile relativa.[2][10][8]
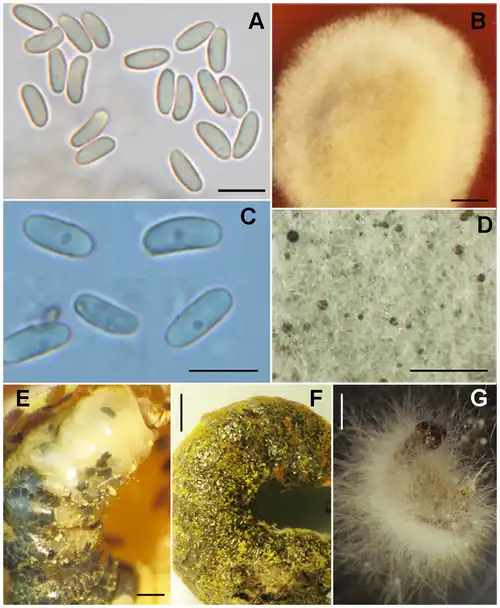
Growth, morphology and pathobiology
A. aggregata is an obligate parasite that can cause chalkbrood by the fifth instar.[5] The majority of the life cycle and growth of A. aggregata occurs in M. rotundata larvae. Infection of bee larvae occurs only via ingestion of resting spores,[3] and is not possible via spore inhalation nor contact with the fungal vegetative form.[6][5] Spores develop in the larva and cause it to swell, bursting the larval integument (giving the dead larvae a ragged appearance)[1] and furthering the spread of the fungus. Buildup of larval cadavers traps the unaffected emerging bees, forcing them to chew through the cadavers and be covered in spores.[7] Bees covered in spores then contaminate food provisions for other broods[7] and spread the infection.
Early vegetative growth utilizes gut lumen nutrients.[6] A. aggregata grows through the midgut wall to the hemocoele (event trigger is unknown, not because of lack of space nor food)[6] eventually replacing larval tissue.[3] Resulting larva are filled with a mycelial mat comprising two layers: a dense inner layer and a less dense outer layer.[6]
Sexual development
Ascospore morphology consists of two layers: an inner chitinous and smooth layer, and an outer layer that is rough, spotted,[1] and not composed of chitin nor cellulose.;[6]) Ascospore development in A. aggregata is very unique and the resulting structure is referred to as a "spore cyst", or "ascocyst" or "synascus".[8] Sexual development occurs on the outer mycelial mat in the subcuticular region,[3][6] and is documented to proceed as follows:
- The vegetative hyphae tips swell and form a thallus[6]
- The middle of the thallus grows and forms a nutriocyte (previously referred to as an archicarp[8])
- The apical portion differentiates into the trichogyne cell.[6]
- Compatible trichogyne fuse and initiate plasmogamy.[6]
- Resulting dikaryotic fungal protoplasm then enters the nutriocyte and causes enlargement of the nutriocyte.[6]
- Nutriocyte growth causes the integument to rupture and initiate development of a fragile spherical structure without a cell wall.[6]
- Individual spores then pack together into a seemingly membrane-less spore ball.[11]
- Multiple spore balls then join and form a spore cyst.[11]
- Cell wall deposition changes spore colour from opaque white to grey to dull black[3][6]
Physiology
A. aggregata has been found to be unable to break down chitin.[6][3]
Diagnostic Considerations
Although ascospore development is very unique, it is very hard to identify A. aggregata because the spore balls and conidia tend to resemble other species.[12] Recent investigations by James and Skinner (2005)[12] have discovered that PCR of the ITS domain of ribosomal DNA with species specific primer sets allows the detection of fungal DNA (working, even, in asymptomatic individuals).[12] The PCR technique can also be used on hair and honey samples to avoid the difficulty of culturing spores,[12] as spore were shown before to only germinate well in lipids.[13] Storage of the fungus has also proven to be difficult as it collapses after 1–2 months during normal culture passaging.[14] However, Jensen et al. (2009) found that spores could be preserved via cryopreservation or freeze-drying whereas hyphae unfortunately could not be preserved.[14]
Economic importance
M. rotundata is the primary pollinator of the commercially grown alfalfa seed,[11][7] accounting for 46,000 metric tonnes of North American alfafa seed (two-thirds the global production) in 2004.[7] M. rotundata is also the second most valuable field crop pollinator, behind the honey bee, because of the value of alfalfa in animal feed and hay.[7] A. aggregata has been killing this economic pollinator in the US since 1972[10] and has been reported to be able to kill greater than 50% of a population.[8]
Effective management of the fungus has yet to be discovered, as the current registered treatment in Canada (paraformaldehyde fumigation of spores[7]) involves a carcinogen and other treatment options (heat and chloride treatments) are expensive and labour-intensive.[7]
References
- 1 2 3 4 Skou, Jens-Peder (1975). "Two new species of ascosphaera and notes on the conidial state of Bettsia Alvei". Friesia. 11 (1): 62–74.
- 1 2 3 Goulson, Dave (2010). Bumblebees : behaviour, ecology, and conservation (2nd ed.). New York: Oxford University Press. p. 230. ISBN 978-0-19-955306-8.
- 1 2 3 4 5 6 7 Capinera, John L. (2008). Encyclopedia of entomology (2nd ed.). Springer. p. 304. ISBN 978-1-4020-6242-1.
- ↑ Tanada, Yoshinori; Kaya, Henry K. (1993). Insect pathology. California: Academic Press. p. 349. ISBN 0-12-683255-2.
- 1 2 3 4 Vandenberg, John D.; Stephen, W.P. (March 1982). "Etiology and symptomatology of chalkbrood in the alfalfa leafcutting bee, Megachile rotundata". Journal of Invertebrate Pathology. 39 (2): 133–137. doi:10.1016/0022-2011(82)90002-7.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 McManus, William R.; Youssef, Nabil N. (September 1984). "Life Cycle of the Chalk Brood Fungus, Ascosphaera aggregata, in the Alfalfa Leafcutting Bee, Megachile rotundata, and Its Associated Symptomatology". Mycologia. 76 (5): 830–842. doi:10.2307/3793139. JSTOR 3793139.
- 1 2 3 4 5 6 7 8 Pitts-Singer, Theresa L.; Cane, James H. (7 January 2011). "The Alfalfa Leafcutting Bee, Megachile rotundata: The World's Most Intensively Managed Solitary Bee". Annual Review of Entomology. 56 (1): 221–237. doi:10.1146/annurev-ento-120709-144836. PMID 20809804.
- 1 2 3 4 5 6 7 Bissett, John (December 1988). "Contribution toward a monograph of the genus". Canadian Journal of Botany. 66 (12): 2541–2560. doi:10.1139/b88-346.
- ↑ Rank, GH; Goerzen, DW (1981). "Native leafcutter bee species and associated parasites in commercial hives in Saskatchewan, Canada". Apidologie. 12 (3): 211–220. doi:10.1051/apido:19810301.
- 1 2 Goerzen, D.W.; Erlandson, M.A.; Bissett, J. (31 May 2012). "Occurrence of Chalkbrood Caused by Ascosphaera Aggregata Skou in a Native Leafcutting Bee, Megachile Relativa Cresson (Hymenoptera: Megachilidae), in Saskatchewan". The Canadian Entomologist. 122 (6): 1269–1270. doi:10.4039/Ent1221269-11. S2CID 85760024.
- 1 2 3 Richards, K.W. (31 May 2012). "Detection of a Chalkbrood Fungus, Ascosphaera Aggregata, in Larvae of the Alfalfa Leafcutter Bee (Hymenoptera: Megachilidae) from Western Canada". The Canadian Entomologist. 117 (9): 1143–1145. doi:10.4039/Ent1171143-9. S2CID 84474941.
- 1 2 3 4 James, R. R.; Skinner, J. S. (1 October 2005). "PCR diagnostic methods for Ascosphaera infections in bees". Journal of Invertebrate Pathology. 90 (2): 98–103. doi:10.1016/j.jip.2005.08.004. ISSN 0022-2011. PMID 16214164.
- ↑ James, R. R.; Buckner, J. S. (1 October 2004). "Lipids stimulate spore germination in the entomopathogenic ascomycete Ascosphaera aggregata". Mycopathologia. 158 (3): 293–302. doi:10.1007/s11046-004-2910-5. ISSN 1573-0832. PMID 15645171. S2CID 21838968.
- 1 2 Jensen, A. B.; James, R. R.; Eilenberg, J. (1 June 2009). "Long-term storage of Ascosphaera aggregata and Ascosphaera apis, pathogens of the leafcutting bee (Megachile rotundata) and the honey bee (Apis mellifera)". Journal of Invertebrate Pathology. 101 (2): 157–160. doi:10.1016/j.jip.2009.03.004. ISSN 0022-2011. PMID 19332075.