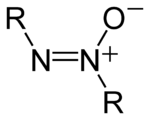
In chemistry, azoxy compounds are a group of organic compounds sharing a common functional group with the general structure R−N=N+(−O−)−R.[1] They are considered N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles and cycloadd to double bonds. Most azoxy-containing compounds have aryl substituents.
Preparation
Azoxybenzene and its derivatives are typically prepared by reduction of nitrocompounds, such as the reduction of nitrobenzene with arsenous oxide.[2] Such reactions are proposed to proceed via the intermediacy of the nitroso compounds and hydroxylamines, e.g. phenylhydroxylamine and nitrosobenzene (Ph = phenyl, C6H5):[3]: 445
Nitrosocarbamate esters decarboxylate in strong base to an azotate susceptible to strong alkylation agents:
- –N(H)CO2R + 2NO2 → –N(N=O)CO2R + HNO3
- –N(N=O)CO2R + KOR → –N=NO−K+ + CO2 + R2O
- –N=NO−K+ + R3O+BF−
4 → –N(N=O)R + R2O + KBF4
An alternative route involves oxidation of azobenzenes with peroxy acids.[3]: 96–100
Structure
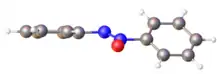
Azoxybenzene compounds are more stable as their trans isomer isomer. In Ph2N2O, the N-N and N-O bond lengths are is 1.24 and 1.255 Å respectively, corresponding to some double bonds character. The CNNC and CNNO are near 176°.[4]
Trans-azoxydibenzene's resonance form with a negative formal charge on oxygen (–N=N+(O−)–) corresponds to a theoretical 6‑D dipole moment. However, the observed moment is only 4.7 D, suggesting a substantial resonance contribution in which the other nitrogen bears negative charge (–N−–N+(=O)–).[5]: 42
Reactions
Under ultraviolet light transazobenzene compounds isomerize to their cis isomers, analogous to azobenzene. Similar reaction conditions can instead cause isomery to an ortho-azophenol, or migration of the oxygen atom across the two nitrogens.[3]: 101, 766, 1008
Unlike azo compounds, azoxy compounds do not fragment thermally to lose nitrous oxide; the process is believed forbidden by orbital symmetries. Correspondingly, the reaction is possible under UV light with wavelength approximately 220 nm.[3]: 922, 1007
The azoxy group is electron-withdrawing, but in non-oxidizing media, aliphatic α hydrogens must be situated between two azoxy groups to appreciably dissociate. Alkyllithia replace the hydrogens, but with reduction:
- –N+(O−)=NC(H)< + 2LiR → –N=NC(R)< + Li2O↓ + RH
Basic oxidants abstract α hydrogens in a free-radical process, leading to dimerization.[3]: 712–716
Azoxyarenes ortho to a benzylic carbon with good leaving group eliminate the leaving group to give an indazolone (the Davis-Beirut reaction).[3]: 835–836
Azoxy compounds are weak bases, and unstable to strong acids. Azoxyarenes undergo the Wallach rearrangement to para-azophenols; primary and secondary azoxyaliphates tautomerize to a hydrazamide.[3]: 173–174, 621, 837
The two aryl groups in azoxyarenes undergo electrophilic aromatic substitution differently. In the case of azoxybenzene, PhNN(O)– reacts at the meta position, while PhN(O)N– reacts at the ortho and para positions.[3]: 247,712–713
Electrochemical reduction converts azoxyarenes to azo compounds. Single-electron reductants give a deep-blue radical anion, which dimerizes in aqueous solution to the corresponding azo compound and hydrogen peroxide. Strong reducing agents such as lithium aluminum hydride also hydrogenolyze electronegative ortho or para arene substituents, but catalytic hydrogenation selects out the azoxy link. Conversely, sodium borohydride preserves the azoxy group even as it reduces arene substituents.[3]: 452–455, 616–617, 619–620, 924–925
Safety
Alkyl azoxy compounds, e.g. azoxymethane are suspected to be genotoxic.[6]
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "azoxy compounds". doi:10.1351/goldbook.A00567
- ↑ H. E. Bigelow and Albert Palmer "Azoybenzene" Org. Synth. 1931, 11, 16. doi:10.15227/orgsyn.011.0016
- 1 2 3 4 5 6 7 8 9 Patai, Saul, ed. (1975-01-01). The Chemistry of the Hydrazo, Azo and Azoxy Groups. The Chemistry of Functional Groups. Vol. 1. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/0470023414. ISBN 978-0-471-66924-1.
- 1 2 González Martínez, Sandra Patricia; Bernès, Sylvain (2007). "trans -Diphenyldiazene Oxide". Acta Crystallographica Section E: Structure Reports Online. 63 (8): o3639. Bibcode:2007AcCrE..63O3639G. doi:10.1107/S1600536807035787.
- ↑ Patai, Saul, ed. (1977-03-16). Double-Bonded Functional Groups. The Chemistry of Functional Groups. Vol. 1. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/9780470771501. ISBN 978-0-470-77150-1.
- ↑ Guideline On The Limits Of Genotoxic Impurities "Archived copy" (PDF). Archived from the original (PDF) on 2006-09-04. Retrieved 2007-07-11.
{{cite web}}: CS1 maint: archived copy as title (link)