Bromobenzenes are a group of aryl bromides/halobenzenes consisting of one or more bromine atoms as substituents on a benzene core. They have the formula C6H6–nBrn, where n = 1–6 is the number of bromine atoms. Depending on the number of bromine substituents, there may be several constitutional isomers possible.
Isomers
- Monobromobenzene
- Dibromobenzene
- Tribromobenzene
- 1,2,3-Tribromobenzene
- 1,2,4-Tribromobenzene
- 1,3,5-Tribromobenzene
- Tetrabromobenzene
- 1,2,3,4-Tetrabromobenzene
- 1,2,3,5-Tetrabromobenzene
- 1,2,4,5-Tetrabromobenzene
- Pentabromobenzene
- Hexabromobenzene
Preparation
Bromobenzenes may be prepared by electrophilic aromatic bromination of benzene and benzene derivatives, using elemental bromine and the Lewis acid catalyst iron(III) bromide. They may also be prepared from diazonium compounds.
Reactions
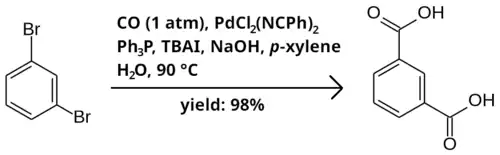
Bromobenzenes may be carboxylated to carboxylic acids with carbon monoxide. The reaction takes place in a two-phase mixture of p-xylene as solvent, in the presence of catalytic PdCl
2(NCPh)
2 and triphenylphosphine (PPh
3), tetrabutylammonium iodide (TBAI) as a phase-transfer catalyst, and sodium hydroxide as a base. Below is an example reaction of 1,3-dibromobenzene to isophthalic acid.[1]
References
- ↑ Panek, J.S. (2006). "Synthesis from Organic Halides". Category 3, Compounds with Four and Three Carbon Heteroatom Bonds. Science of Synthesis. Vol. 20a. Georg Thieme Verlag KG. p. 141. doi:10.1055/sos-SD-020-00102. ISBN 9783131187116.