 | |
| Names | |
|---|---|
| Preferred IUPAC name
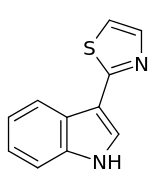
3-(1,3-Thiazol-2-yl)-1H-indole | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.236.489 |
PubChem CID |
|
| |
| |
| Properties | |
| C11H8N2S | |
| Molar mass | 200.26 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens.[1]
Structure
The base structure of camalexin consists of an indole ring derived from tryptophan. The ethanamine moiety attached to the 3 position of the indole ring is subsequently rearranged into a thiazole ring.
Biosynthesis
While the biosynthesis of camalexin in planta has not been fully elucidated, most of the enzymes involved in the pathway are known and involved in a metabolon complex.[2] The pathway starts with a tryptophan precursor which is subsequently oxidized by two cytochrome P450 enzymes.[3] The indole-3-acetaldoxime is then converted to indole-3-acetonitrile by another cytochrome P450, CYP71A13.[1] A glutathione conjugate followed by a subsequent unknown enzyme is needed to form dihydrocamalexic acid.[4][5] A final decarboxylation step by cytochrome P450 CYP71B15, also called phytoalexin deficient4 (PAD3) results in the final product, camalexin.[6][7]
Biological activity
Camalexin is cytotoxic against aggressive prostate cancer cell lines in vitro.[8]
References
- 1 2 Nafisi, Majse; Goregaoker, Sameer; Botanga, Christopher J.; Glawischnig, Erich; Olsen, Carl E.; Halkier, Barbara A.; Glazebrook, Jane (2007). "Arabidopsis Cytochrome P450 Monooxygenase 71A13 Catalyzes the Conversion of Indole-3-Acetaldoxime in Camalexin Synthesis". The Plant Cell. 19 (6): 2039–2052. doi:10.1105/tpc.107.051383. PMC 1955726. PMID 17573535.
- ↑ Mucha, Stefanie; Heinzlmeir, Stephanie; Kriechbaumer, Verena; Strickland, Benjamin; Kirchhelle, Charlotte; Choudhary, Manisha; Kowalski, Natalie; Eichmann, Ruth; Hueckelhoven, Ralph; Grill, Erwin; Kuster, Bernhard; Glawischnig, Erich (2019). "The formation of a camalexin-biosynthetic metabolon". The Plant Cell. 31 (11): 2697–2710. doi:10.1105/tpc.19.00403. PMC 6881122. PMID 31511315.
- ↑ Glawischnig, E.; Hansen, B. G.; Olsen, C. E.; Halkier, B. A. (2004). "Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis". Proceedings of the National Academy of Sciences. 101 (21): 8245–8250. Bibcode:2004PNAS..101.8245G. doi:10.1073/pnas.0305876101. PMC 419588. PMID 15148388.
- ↑ Su, Tongbing; Xu, Juan; Li, Yuan; Lei, Lei; Zhao, Luo; Yang, Hailian; Feng, Jidong; Liu, Guoqin; Ren, Dongtao (2011). "Glutathione-Indole-3-Acetonitrile is Required for Camalexin Biosynthesis in Arabidopsis thaliana". The Plant Cell. 23 (1): 364–380. doi:10.1105/tpc.110.079145. PMC 3051237. PMID 21239642.
- ↑ Geu-Flores, Fernando; Møldrup, Morten Emil; Böttcher, Christoph; Olsen, Carl Erik; Scheel, Dierk; Halkier, Barbara Ann (2011). "Cytosolic γ-Glutamyl Peptidases Process Glutathione Conjugates in the Biosynthesis of Glucosinolates and Camalexin in Arabidopsis". The Plant Cell. 23 (6): 2456–2469. doi:10.1105/tpc.111.083998. PMC 3160024. PMID 21712415.
- ↑ Zhou, Nan; Tootle, Tina L.; Glazebrook, Jane (1999). "Arabidopsis PAD3, a Gene Required for Camalexin Biosynthesis, Encodes a Putative Cytochrome P450 Monooxygenase". The Plant Cell. 11 (12): 2419–2428. doi:10.1105/tpc.11.12.2419. PMC 144139. PMID 10590168.
- ↑ Schuhegger, Regina; Nafisi, Majse; Mansourova, Madina; Petersen, Bent Larsen; Olsen, Carl Erik; Svatoš, Aleš; Halkier, Barbara Ann; Glawischnig, Erich (2006). "CYP71B15 (PAD3) Catalyzes the Final Step in Camalexin Biosynthesis". Plant Physiology. 141 (4): 1248–1254. doi:10.1104/pp.106.082024. PMC 1533948. PMID 16766671.
- ↑ Smith, Basil A.; Neal, Corey L.; Chetram, Mahandranauth; Vo, Baohan; Mezencev, Roman; Hinton, Cimona; Odero-Marah, Valerie A. (2013). "The phytoalexin camalexin mediates cytotoxicity towards aggressive prostate cancer cells via reactive oxygen species". Journal of Natural Medicines. 67 (3): 607–618. doi:10.1007/s11418-012-0722-3. PMC 3644009. PMID 23179315.