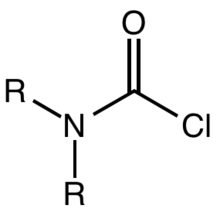
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb.[1]
Production and examples
Carbamoyl chlorides are prepared by the reaction of an amine with phosgene:
- 2 R2NH + COCl2 → R2NCOCl + [R2NH2]Cl
They also arise by the addition of hydrogen chloride to isocyanates:
- RNCO + HCl → RNHCOCl
In this way, carbamoyl chlorides can be prepared with N-H functionality.
Reactions
In a reaction that is typically avoided, hydrolysis of carbamoyl chlorides gives carbamic acids:
- R2NCOCl + H2O → R2NC(O)OH + HCl
Owing to the influence of the amino group, these compounds are less hydrolytically sensitive than the usual acid chlorides. A related but more useful reaction is the analogous reaction with alcohols:[2]
- R2NCOCl + R'OH + C5H5N → R2NC(O)OR' + C5H5NHCl
References
- ↑ Peter Jäger, Costin N. Rentzea and Heinz Kieczka "Carbamates and Carbamoyl Chlorides" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_051
- ↑ Carol Dallaire; Isabelle Kolber; Marc Gingras (2002). "Nickel-Catalyzed Coupling of Aryl O-Carbamates with Grignard Reagents: 2,7-Dimethylnaphthalene". Org. Synth. 78: 42. doi:10.15227/orgsyn.078.0042.). Notes: describes use of diethylcarbamoyl chloride as protecting and leaving group.