 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
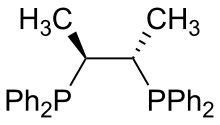
rel-[(2R,3R)-Butane-2,3-diyl]bis(diphenylphosphane) | |||
Other names
| |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.152.152 | ||
PubChem CID |
|||
| UNII |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C28H28P2 | |||
| Molar mass | 426.47 g/mol | ||
| Appearance | White powder | ||
| Melting point | 104 to 109 °C (219 to 228 °F; 377 to 382 K) | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
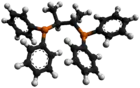
Chiraphos is a chiral diphosphine employed as a ligand in organometallic chemistry. This bidentate ligand chelates metals via the two phosphine groups. Its name is derived from its description — being both chiral and a phosphine. As a C2-symmetric ligand, chiraphos is available in two enantiomeric forms, S,S and R,R, each with C2 symmetry.
Preparation
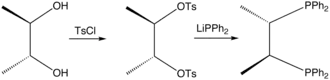
Chiraphos is prepared from S,S or R,R-2,3-butanediol, which are derived from commercially available S,S or R,R-tartaric acid; the technique of using cheaply available enantiopure starting materials is known as chiral pool synthesis. The diol is tosylated and then the ditosylate is treated with lithium diphenylphosphide.[1] The ligand was an important demonstration of how the conformation of the chelate ring can affect asymmetric induction by a metal catalyst. Prior to this work, in most chiral phosphines, e.g., DIPAMP, phosphorus was the stereogenic center.
References
- ↑ Fryzuk, M. D.; Bosnich, B. (1977). "Asymmetric synthesis. Production of optically active amino acids by catalytic hydrogenation". Journal of the American Chemical Society. 99 (19): 6262–6267. doi:10.1021/ja00461a014. PMID 893889.