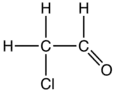
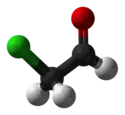
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloroacetaldehyde | |||
| Systematic IUPAC name
Chloroethanal | |||
| Other names
2-Chloroacetaldehyde 2-Chloroethanal | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.158 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3ClO | |||
| Molar mass | 78.50 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | acrid, penetrating[1] | ||
| Density | 1.117 g/mL | ||
| Melting point | −16.3 °C (2.7 °F; 256.8 K) hydrate melts at 43–50 °C[1] | ||
| Boiling point | 85 to 85.5 °C (185.0 to 185.9 °F; 358.1 to 358.6 K) | ||
| soluble[1] | |||
| Solubility | organic solvents | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
alkylating agent | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H301, H311, H314, H330, H351, H400 | |||
| Flash point | 87.7 °C (189.9 °F) (closed cup) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
89 mg/kg (oral, rat) 82 mg/kg (oral, mouse)[2] | ||
LC50 (median concentration) |
200 ppm (rat, 1 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
C 1 ppm (3 mg/m3)[4] | ||
REL (Recommended) |
C 1 ppm (3 mg/m3)[4] | ||
IDLH (Immediate danger) |
45 ppm[4] | ||
| Related compounds | |||
Related compounds |
2-chloroethanol, Chloroacetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
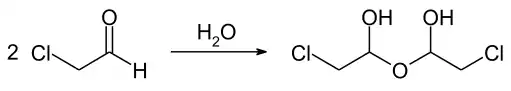
Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.
Chloroacetaldehyde is a metabolite of the antineoplastic ifosfamide and believed to be responsible for some of the toxicity observed with ifosfamide.
Synthesis and occurrence
Hydrated chloroacetaldehyde is produced by the chlorination of aqueous vinyl chloride:
- ClCH=CH2 + Cl2 + H2O → ClCH2CHO + 2 HCl
It can also be prepared from vinyl acetate[5] or by careful chlorination of acetaldehyde.[1] The related bromoacetaldehyde is prepared via bromination of vinyl acetate. It also rapidly forms an acetals in the presence of alcohols.[6]
Water free chloroacetaldehyde is prepared from the hydrate by azeotropic distillation with chloroform, toluene, or carbon tetrachloride. Anhydrous chloroacetaldehyde reversibly converts to polyacetals.[7][1] Less reactive chloroacetaldehyde derivatives might be used instead to obtain chloroacetaldehyde or bypass its intermediate formation completely: e.g. chloroacetaldehyde dimethyl acetal (2-chloro-1,1-dimethoxyethane) hydrolyzes in acidic conditions to give chloroacetaldehyde, which may then quickly react with the other reagents[7] instead of polymerizing.
Relevant to its occurrence in humans, it arises via the isomerization of chloroethylene oxide, a metabolite of vinyl chloride.[8]
Reactions
Chloroacetaldehyde readily hydrates:
Being bifunctional, chloroacetaldehyde is a precursor to many heterocyclic compounds. It condenses with thiourea derivatives to give aminothiazoles. This reaction was once used in the preparation of sulfathiazole, one of the first sulfa drugs.[5] Chloroacetaldehyde is a building block in the synthesis of the pharmaceuticals altizide, polythiazide, brotizolam, and ciclotizolam.[7] Chloroacetaldehyde is an alkylating agent. It reacts with adenosine and cytidine to give cyclic products containing a fused imidazole group. This reaction is related to the possible mutagenic properties of chloroacetaldehyde.[9]
Environmental aspects
Chloroacetaldehyde is a metabolite in the degradation of 1,2-dichloroethane, which initially converts to chloroethanol. This metabolic pathway is topical because 1,2-dichloroethane is produced on a large scale as a precursor to vinyl chloride.[10]
Safety
Chloroacetaldehyde is corrosive to mucous membranes. It irritates eyes, skin and respiratory tract.[1]
Based on data collected from human studies in 1962, exposures to 45 ppm of chloroacetaldehyde were found to be disagreeable and caused conjunctival irritation to the subjects.[11] The Occupational Safety and Health Administration established a permissible exposure limit at a ceiling of 1 ppm (3 mg/m3) for exposures to chloroacetaldehyde.[12]
References
- 1 2 3 4 5 6 The Merck index. S Budavari, M O'Neil, A Smith (12 ed.). Merck. 1996. p. 2108. ISBN 9780911910124.
{{cite book}}: CS1 maint: others (link) - ↑ "Chloroacetaldehyde". National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 20 February 2015.
- ↑ "Chloroacetaldehyde". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0118". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Jira, Reinhard; Kopp, Erwin; McKusick, Blaine C.; Röderer, Gerhard; Bosch, Axel; Fleischmann, Gerald (2007). "Chloroacetaldehydes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_527.pub2. ISBN 978-3527306732.
- ↑ S. M. McElvain and D. Kundiger "Bromoacetal" Organic Syntheses 1943, volume 23, p. 8. doi:10.15227/orgsyn.023.0008.
- 1 2 3 Keiji, T (1992-10-30). "α-Chlorocarbonyl Compounds: Their Synthesis and Applications (Commemoration Issue Dedicated to Professor Shigeo Tanimoto On the Occasion of His Retirement)". Bulletin of the Institute for Chemical Research, Kyoto University. 70 (3): 341. hdl:2433/77455. ISSN 0023-6071.
- ↑ Swenberg, J. A.; Lu, K.; Moeller, B. C.; Gao, L.; Upton, P. B.; Nakamura, J.; Starr, T. B. (2011). "Endogenous versus Exogenous DNA Adducts: Their Role in Carcinogenesis, Epidemiology, and Risk Assessment". Toxicological Sciences. 120 (Suppl 1): S130–S145. doi:10.1093/toxsci/kfq371. PMC 3043087. PMID 21163908.
- ↑ Singer, B. (1996). "DNA Damage: Chemistry, Repair, and Mutagenic Potential". Regulatory Toxicology and Pharmacology. 23 (1 Pt 1): 2–13. doi:10.1006/rtph.1996.0002. PMID 8628915.
- ↑ Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and Biochemistry of 1,2-Dichloroethane Degradation", Biodegradation, 1994, 5, 249-57.doi:10.1007/BF00696463
- ↑ Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards