Kit Cummins | |
|---|---|
| Born | Christopher Colin Cummins February 28, 1966 Boston, Massachusetts, US |
| Alma mater | |
| Awards |
|
| Scientific career | |
| Fields | Inorganic Chemistry, Main Group Chemistry |
| Institutions | Massachusetts Institute of Technology |
| Thesis | Synthetic investigations featuring amidometallic complexes (1993) |
| Doctoral advisor | Richard R. Schrock |
| Other academic advisors | Peter T. Wolczanski |
| Doctoral students | Brandi Cossairt, Jonas C. Peters |
| Other notable students |
|
| Website | ccclab |
Christopher "Kit" Colin Cummins (born February 28, 1966) is an American chemist, currently the Henry Dreyfus Professor at the Massachusetts Institute of Technology. He has made contributions to the coordination chemistry of transition metal nitrides, phosphides, and carbides.[2][3]
Early life and education
Cummins was born in Boston, Massachusetts, on February 28, 1966.[4] He attended Middlebury College and Stanford University before transferring to Cornell University where he performed undergraduate research under the direction of Peter T. Wolczanski.[5] At Cornell, Cummins conducted research on the reactivity of low-coordinate zirconium and titanium complexes bearing bulky silanamide ligands (tBu3SiNH−), with small molecules such as methane, benzene, and carbon monoxide.[6][7][8]
After graduating from Cornell with an AB degree in 1989, Cummins went to the Massachusetts Institute of Technology to obtain his PhD in chemistry in 1993 under the direction of Richard R. Schrock.[9] Cummins conducted doctoral research on the synthesis of low-coordinate transition metal complexes bearing trialkylsilated variants of the tris(2-aminoethyl)amine ligand.[10][11][12][13][14] In collaboration with Robert E. Cohen, he also discovered a new technique for synthesizing nanoclusters of metal sulfide semiconductors within block copolymer microdomains.[15][16]
Independent career
After receiving his PhD in 1993, Cummins was invited to stay at MIT as an assistant professor and was later promoted to full professor in 1996. Cummins became the Henry Dreyfus Professor in Chemistry in 2015.[17]
Research
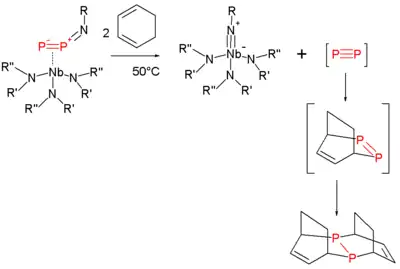
In one contribution, Cummins and coworkers described routes to simple phosphorus compounds including a low temperature route to diphosphorus:[18]
Honors and awards
In 2007, Cummins was awarded the 2007 Raymond and Beverly Sackler Prize in the Physical Sciences by Tel Aviv University[19] and the 2007 F. Albert Cotton Award by the American Chemical Society.[20]
In 2008, Cummins was elected a fellow of the American Academy of Arts & Sciences.[21]
In 2013, Cummins was awarded the Ludwig Mond Award by the Royal Society of Chemistry.[22]
In 2017, Cummins was elected as a member of the National Academy of Sciences.[23] In the same year, the American Chemical Society awarded Cummins the 2017 Linus Pauling Medal in recognition of his synthetic and mechanistic studies of early-transition metal complexes.[24]
References
- ↑ "Polly Arnold – EPSRC website". epsrc.ukri.org. Archived from the original on April 14, 2019. Retrieved February 9, 2019.
- ↑ "Christopher Cummins". Massachusetts Institute of Technology. Archived from the original on May 5, 2017. Retrieved May 1, 2017.
- ↑ "Christopher Cummins". Massachusetts Institute of Technology. Retrieved May 1, 2017.
- ↑ "Member Directory - Christopher C. Cummins". National Academy of Sciences.
- ↑ "Professor Christopher C. Cummins". Massachusetts Institute of Technology. Retrieved March 17, 2023.
- ↑ Cummins, Christopher C.; Baxter, Steven M.; Wolczanski, Peter T. (1988). "Methane and benzene activation via transient (tert-Bu3SiNH)2Zr:NSi-tert-Bu3". Journal of the American Chemical Society. 110 (26): 8731–8733. doi:10.1021/ja00234a044. ISSN 0002-7863.
- ↑ Cummins, Christopher C.; Van Duyne, Gregory D.; Schaller, Christopher P.; Wolczanski, Peter T. (1991). "Carbonylation of zirconium complex [tert-Bu3SiNH]3ZrH and x-ray structural study of [tert-Bu3SiNH]3ZrCH3". Organometallics. 10 (1): 164–170. doi:10.1021/om00047a044. ISSN 0276-7333.
- ↑ Cummins, Christopher C.; Schaller, Christopher P.; Van Duyne, Gregory D.; Wolczanski, Peter T.; Chan, A. W. Edith; Hoffmann, Roald (1991). "Tri-tert-butylsilyl)imido complexes of titanium: benzene carbon-hydrogen activation and structure of [(tert-Bu3SiNH)Ti]2(.mu.-NSi-tert-Bu3)2". Journal of the American Chemical Society. 113 (8): 2985–2994. doi:10.1021/ja00008a029. ISSN 0002-7863.
- ↑ Cummins, Christopher Colin (1993). Synthetic investigations featuring amidometallic complexes (PhD. thesis). Massachusetts Institute of Technology. hdl:1721.1/12718. OCLC 28863744.
- ↑ Cummins, Christopher C.; Schrock, Richard R.; Davis, William M. (April 1, 1992). "Synthesis of vanadium and titanium complexes of the type RM[(Me3SiNCH2CH2)3N] (R = Cl, alkyl) and the structure of ClV[(Me3SiNCH2CH2)3N]". Organometallics. 11 (4): 1452–1454. doi:10.1021/om00040a011. ISSN 0276-7333.
- ↑ Cummins, Christopher C.; Lee, Jenny; Schrock, Richard R.; Davis, William D. (1992). "Trigonal-Monopyramidal MIII Complexes of the Type [M(N3N)] (M = Ti, V, Cr, Mn, Fe; N3N = [(tBuMe2Si)NCH2CH2]3N)". Angewandte Chemie International Edition in English. 31 (11): 1501–1503. doi:10.1002/anie.199215011. ISSN 1521-3773.
- ↑ Cummins, Christopher C.; Schrock, Richard R.; Davis, William M. (1993). "Phosphinidenetantalum(V) Complexes of the Type [(N3N)Ta=PR] as Phospha-Wittig Reagents". Angewandte Chemie International Edition in English. 32 (5): 756–759. doi:10.1002/anie.199307561. ISSN 1521-3773.
- ↑ Cummins, C. C.; Schrock, R. R. (1994). "Synthesis of an iron(IV) cyanide complex that contains the triamido amine ligand [(tert-BuMe2SiNCH2CH2)3N]3-". Inorganic Chemistry. 33 (2): 395–396. doi:10.1021/ic00080a033. ISSN 0020-1669.
- ↑ Cummins, Christopher C.; Schrock, Richard R.; Davis, William M. (March 1, 1994). "Synthesis of Terminal Vanadium(V) Imido, Oxo, Sulfido, Selenido, and Tellurido Complexes by Imido Group or Chalcogenide Atom Transfer to Trigonal Monopyramidal V[N3N] (N3N = [(Me3SiNCH2CH2)3N]3-)". Inorganic Chemistry. 33 (7): 1448–1457. doi:10.1021/ic00085a038. ISSN 0020-1669.
- ↑ Cummins, C. C.; Beachy, M. D.; Schrock, R. R.; Vale, M. G.; Sankaran, V.; Cohen, R. E. (November 1, 1991). "Synthesis of norbornenes containing tin(II), tin(IV), lead(II), and zinc(II) and their polymerization to give microphase-separated block copolymers". Chemistry of Materials. 3 (6): 1153–1163. doi:10.1021/cm00018a036. ISSN 0897-4756.
- ↑ Cummins, C. C.; Schrock, R. R.; Cohen, R. E. (1992). "Synthesis of zinc sulfide and cadmium sulfide within ROMP block copolymer microdomains". Chemistry of Materials. 4 (1): 27–30. doi:10.1021/cm00019a011. ISSN 0897-4756.
- ↑ "Three Faculty named to Chairs". Massachusetts Institute of Technology. March 13, 2015. Archived from the original on September 16, 2015. Retrieved May 12, 2018.
- ↑ Piro, Nicholas A.; Figueroa, Joshua S.; McKellar, Jessica T.; Cumnins, Christopher C. (September 1, 2006). "Triple-Bond Reactivity of Diphosphorus Molecules". Science. 313 (5791): 1276–1279. Bibcode:2006Sci...313.1276P. doi:10.1126/science.1129630. PMID 16946068. S2CID 27740669. Retrieved July 21, 2013.
- ↑ Wang, Linda (August 20, 2007). "Sackler Prize Winners Announced". Chemical & Engineering News. Vol. 85, no. 34. pp. 75–76.
- ↑ Dagani, Ron (January 15, 2007). "F. Albert Cotton Award in Synthetic Inorganic Chemistry". Chemical & Engineering News. Vol. 85, no. 3. p. 60.
- ↑ Rovner, Sophie L. (April 29, 2008). "American Academy Of Arts & Sciences Elects New Fellows". Chemical & Engineering News.
- ↑ "Cummins selected for RSC Ludwig Mond Award". Massachusetts Institute of Technology. April 29, 2013. Archived from the original on September 15, 2015. Retrieved May 12, 2018.
- ↑ "News from the National Academy of Sciences". National Academy of Sciences. May 2, 2017.
- ↑ Randall, Danielle (June 28, 2017). "Kit Cummins awarded the American Chemical Society Pauling Medal". Massachusetts Institute of Technology.