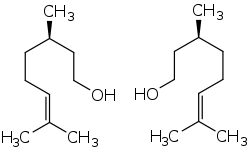
 (+)-Citronellol (left) and (−)-citronellol (right) | |
-Citronellol_3D_ball.png.webp) R-(+)-Citronellol | |
-Citronellol_3D_ball.png.webp) S-(−)-Citronellol | |
| Names | |
|---|---|
| IUPAC name
3,7-Dimethyloct-6-en-1-ol | |
| Other names
(±)-β-Citronellol; Cephrol, Corol | |
| Identifiers | |
3D model (JSmol) |
|
| 1362474 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.069 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H20O | |
| Molar mass | 156.269 g·mol−1 |
| Density | 0.855 g/cm3 |
| Boiling point | 225 °C (437 °F; 498 K) |
| Viscosity | 11.1 mPa s |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H315, H317, H319 | |
| P261, P264, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362, P363, P391, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. (+)-Citronellol, which is found in citronella oils, including Cymbopogon nardus (50%), is the more common isomer. (−)-Citronellol is widespread, but particularly abundant in the oils of rose (18–55%) and Pelargonium geraniums.[1]
Preparation
Several million kilograms of citronellol are produced annually. It is mainly obtained by hydrogenation of geraniol or nerol over copper chromite catalyst.[2] Homogeneous catalysts are used for the production of enantiomers.[3][4]
Uses
Citronellol is used in perfumes and as a fragrance in cleaning products. In many applications, one of the enantiomers is preferred. It is a component of citronella oil, an insect repellant.[2]
Citronellol is used as a raw material for the production of rose oxide.[2][5] It is also a precursor to many commercial and potential fragrances such as citronellol acetate, citronellyl oxyacetaldehyde, citronellyl methyl acetal, and ethyl citronellyl oxalate.[2]
Health and safety
The United States FDA considers citronellol as generally recognized as safe (GRAS) for food use.[6] Citronellol is subject to restrictions on its use in perfumery,[7] as some people may become sensitised to it, but the degree to which citronellol can cause an allergic reaction in humans is disputed.[8][9]
In terms of dermal safety, citronellol has been evaluated as an insect repellent.[10]
See also
References
- ↑ Lawless, J. (1995). The Illustrated Encyclopedia of Essential Oils. ISBN 978-1-85230-661-8.
- 1 2 3 4 Sell, Charles S. (2006). "Terpenoids". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2005181602120504.a01.pub2. ISBN 0471238961.
- ↑ Morris, Robert H. (2007). "Ruthenium and Osmium". In De Vries, J. G.; Elsevier, C. J. (eds.). The Handbook of Homogeneous Hydrogenation. Weinheim: Wiley-VCH. ISBN 978-3-527-31161-3.
- ↑ Ait Ali, M.; Allaoud, S.; Karim, A.; Roucoux, A.; Mortreux, A. (1995). "Catalytic Synthesis of (R)- and (S)-citronellol by homogeneous hydrogenation over amidophosphinephosphinite and diaminodiphosphine rhodium complexes". Tetrahedron: Asymmetry. 6 (2): 369. doi:10.1016/0957-4166(95)00015-H.
- ↑ Alsters, Paul L.; Jary, Walther; Aubry, Jean-Marie (2010). ""Dark" Singlet Oxygenation of β-Citronellol: A Key Step in the Manufacture of Rose Oxide". Organic Process Research & Development. 14: 259–262. doi:10.1021/op900076g.
- ↑ "Redirect". epa.gov. Retrieved 29 July 2015.
- ↑ "Standards Restricted - IFRA International Fragrance Association". Archived from the original on 6 January 2012. Retrieved 19 July 2012.
- ↑ "Cropwatch Report April 2008" (PDF). Archived from the original (PDF) on 10 February 2014. Retrieved 19 July 2012.
- ↑ Survey and health assessment of chemical substances in massage oils Archived 27 September 2007 at the Wayback Machine
- ↑ Taylor, W. G.; Schreck, C. E. (1985). "Chiral-phase capillary gas chromatography and mosquito repellent activity of some oxazolidine derivatives of (+)- and (−)-citronellol". Journal of Pharmaceutical Sciences. 74 (5): 534–539. doi:10.1002/jps.2600740508. PMID 2862274.
