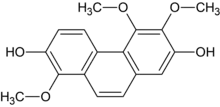
 Chemical structure of confusarin | |
| Names | |
|---|---|
| Preferred IUPAC name
1,5,6-Trimethoxyphenanthrene-2,7-diol | |
| Other names
2,7-dihydroxy-3,4,8-trimethoxyphenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C17H16O5 | |
| Molar mass | 300.30 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Confusarin is a phenanthrenoid found in the orchids Eria confusa[1] and Bulbophyllum reptans.[2] It can also be synthesized.[3]
References
- ↑ Confusarin and confusaridin two phenanthrene derivatives of the orchid Eria Confusa. P.L. Majumder and Amita Kar, Phytochemistry, 1987, Volume 26, Issue 4, Pages 1127–1129, doi:10.1016/S0031-9422(00)82363-8
- ↑ Dimeric phenanthrenes from the orchid Bulbophyllum reptans. P.L Majumder, S Pal and S Majumder, Phytochemistry, Volume 50, Issue 5, 10 March 1999, Pages 891–897, doi:10.1016/S0031-9422(98)00609-8
- ↑ Total synthesis of two natural phenanthrenes: confusarin and a regioisomer. Sylvie Radix and Roland Barret, Tetrahedron, 10 December 2007, Volume 63, Issue 50, Pages 12379–12387, doi:10.1016/j.tet.2007.09.052
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.