 | |
 | |
| Names | |
|---|---|
| Other names
Cuprous bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.210 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CuBr | |
| Molar mass | 143.45 g/mol |
| Appearance | white powder (see text) |
| Density | 4.71 g/cm3, solid |
| Melting point | 492 °C (918 °F; 765 K) |
| Boiling point | 1,345 °C (2,453 °F; 1,618 K) |
| insoluble; slightly soluble in cold water | |
Solubility product (Ksp) |
6.27×10−9[1] |
| Solubility | soluble in HCl, HBr, ammonium hydroxide, acetonitrile negligible in acetone, sulfuric acid |
| −49.0×10−6 cm3/mol | |
Refractive index (nD) |
2.116 |
| 1.46 D | |
| Hazards | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 mg/m3 (as Cu)[2] |
REL (Recommended) |
TWA 1 mg/m3 (as Cu)[2] |
IDLH (Immediate danger) |
TWA 100 mg/m3 (as Cu)[2] |
| Related compounds | |
Other anions |
Copper(I) chloride Copper(I) iodide |
Other cations |
Silver(I) bromide Copper(II) bromide Mercury(I) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
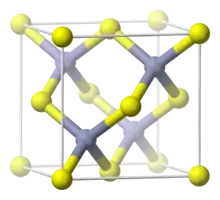
Copper(I) bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.
Preparation, basic properties, structure
The compound is white, although samples are often colored due to the presence of copper(II) impurities.[3] The copper(I) ion also oxidizes easily in air. It is commonly prepared by the reduction of cupric salts with sulfite in the presence of bromide.[4] For example, the reduction of copper(II) bromide with sulfite yields copper(I) bromide and hydrogen bromide:
- 2 CuBr2 + H2O + SO2−
3 → 2 CuBr + SO2−
4 + 2 HBr
CuBr is insoluble in most solvents due to its polymeric structure, which features four-coordinated, tetrahedral Cu centers interconnected by bromide ligands (ZnS structure). Upon treatment with Lewis bases, CuBr converts to molecular adducts. For example, with dimethyl sulfide, the colorless complex is formed:[5]
- CuBr + S(CH3)2 → CuBr(S(CH3)2)
In this coordination complex, the copper is two-coordinate, with a linear geometry. Other soft ligands afford related complexes. For example, triphenylphosphine gives CuBr(P(C6H5)3), although this species has a more complex structure. Thermal excitation of copper(I) bromide vapour yields a blue-violet emission which is of greater saturation than known copper(I) chloride emission.[6] Copper(I) bromide is hence an advantageous emitter in pyrotechnic flames.
Applications in organic chemistry
In the Sandmeyer reaction, CuBr is employed to convert diazonium salts into the corresponding aryl bromides:[4]
- ArN+
2 + CuBr → ArBr + N2 + Cu+
The aforementioned complex CuBr(S(CH3)2) is widely used to generate organocopper reagents.[5] Related CuBr complexes are catalysts for atom transfer radical polymerization and copper-catalyzed cross-dehydrogenative couplings (CDC).
See also
References
- ↑ Rumble, John (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- 1 2 This report gives a procedure for generating CuBr: Hartwell, Jonathan L. (1955). "o-Chlorobromobenzene". Organic Syntheses.; Collective Volume, vol. 3, p. 185
- 1 2 Jarowicki, K.; Kocienski; Qun, L. "1,2-Metallate Rearrangement: (Z)-4-(2-Propenyl)-3-Octen-1-ol". Organic Syntheses. 79: 11.; Collective Volume, vol. 10, p. 662
- ↑ Koch, E.-C. (2015). "Spectral Investigation and Color Properties of Copper(I) Halides CuX (X=F, Cl, Br, I) in Pyrotechnic Combustion Flames". Propellants Explos. Pyrotech. 40 (6): 798–802. doi:10.1002/prep.201500231.