 | |
| Names | |
|---|---|
| Other names
Glycine imine, iminoacetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 1780785 | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C2H3NO2 | |
| Molar mass | 73.051 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
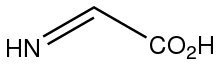
Dehydroglycine is the organic compound with the formula HNCHCO2H. This rarely observed species is invoked as the product of oxidation (dehydrogenation) of glycine by glycine oxidase (ThiO), which is a step in the biosynthesis of thiamin.[1] It is also invoked as a product of the radical SAM-induced fragmentation of tyrosine.[2] It is an imino acid.
References
- ↑ Begley, Tadhg P.; Ealick, Steven E. (2010). "Thiamin Biosynthesis". Comprehensive Natural Products II. pp. 547–559. doi:10.1016/B978-008045382-8.00148-9. ISBN 9780080453828.
- ↑ Britt, R. David; Rao, Guodong; Tao, Lizhi (2020). "Bioassembly of complex iron–sulfur enzymes: Hydrogenases and nitrogenases". Nature Reviews Chemistry. 4 (10): 542–549. doi:10.1038/s41570-020-0208-x. PMC 8023223. PMID 33829110.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.