 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cholesta-5,24-dien-3β-ol | |
| Systematic IUPAC name
(1R,3aS,3bS,7S,9aR,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-6-methylhept-5-en-2-yl]-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.671 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.64 g/mol |
| Appearance | White powder |
| Melting point | 121.5 °C (250.7 °F; 394.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
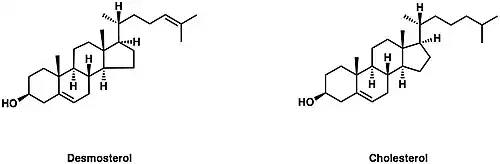
Desmosterol (Cholesta-5,24-dien-3β-ol) is a lipid present in the membrane of phytoplankton and an intermediate product in cholesterol synthesis in mammal cells.[1] Structurally, desmosterol has a similar backbone to cholesterol, with the exception of an additional double bond in the structure of desmosterol.
The similarity can be seen biologically through the synthesis of cholesterol in the human body, as desmosterol is the immediate precursor to cholesterol in the Bloch pathway.[2] Desmosterol is accumulated in desmosterolosis and undergoes reduction with the catalyst 24-dehydrocholesterol reductase to form cholesterol.[3]
In 2014, desmosterol was named the Molecule of the Year 2012 by the International Society for Molecular and Cell Biology and Biotechnology Protocols and Researches (ISMCBBPR).[4]
Background
Desmosterol is classified as a cholestadienol, a subgroup of a wider known group of sterols, which naturally occur in eukaryotes. The presence of desmosterol in oceans and lakes has the potential to diagnose anoxic conditions and to study trends in steroid chemistry during the early stages of diagenesis.[5] Desmosterol has been found in high yields in samples of Rhizosolenia setigera (Brightwell) in Western Svalbard,[6] and from surface sediment off of the Peruvian Shelf sediment-water interface.[5]
In 1955, desmosterol was first described and isolated from chick embryo sterol with a 2% yield and was postulated as being a biological precursor to cholesterol by Stokes et al.[7] The first known isolation of desmosterol in invertebrates was published in 1957 by Fagerlund and Idler.[8] This new sterol was isolated in large amounts from balanus glandula, a barnacle species found on the North American Pacific coast. Additionally, small amounts of desmosterol have been found in other crustaceans such as lobster and shrimp in the homarus americanus and pandalus borealis species. This strengthens the conclusion that desmosterol must be created exogenously, as crustaceans have not been seen to biosynthesise sterols.[9]
Biological occurrence
Desmosterol has been largely found within a large population of marine invertebrates. Major sources include barnacles,[8] red algae,[10] annelida,[11] and molluscs.[12] This has led to the idea of an exogenous origin of desmosterol in phytoplankton. In 1967, desmosterol was also identified in large percentages in the red algae Laurencia pinnatifida, Polusiphonia nigrescens, Porphyra purpurea, and Dulse (Rhodymenia palmata) after previously having been undetected.
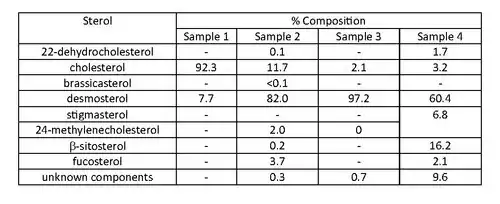
In the 1968 paper by Idler, Saito and Wiseman,[10] dulse samples were analysed and the sterols present were determined by gas liquid chromatography. Samples were collected near Grand Manan Island, New York in 1964 and 1965, and showed a significant difference in the presence of desmosterol. Samples 1 and 2 were from the same source, as were samples 3 and 4, and all samples were harvested at different times. Samples 2, 3, and 4 had the major sterol as desmosterol, compared to sample 1, with the major sterol being cholesterol.[10] This indicates a 70% difference in desmosterol composition in the sterol samples (1 and 2) over a period of one year. There has been no conclusive evidence to show whether seasonal variation of sterols in barnacles occur.
Biosynthesis
There are two major pathways for cholesterol biosynthesis, being the Kandutsch-Russell and Bloch pathways.[13] The Bloch pathway, named after Konrad Bloch, occurs naturally alongside the mevalonate pathway in humans within the cell. The discovery of this pathway led Bloch, joint with Feodor Lynen, to receive a Nobel Prize in Physiology or Medicine in 1964. The award was awarded “for their discoveries concerning the mechanism and regulation of the cholesterol and fatty acid metabolism”.[14]

Preservation
Desmosterol has been seen to be present in marine sponges as a biomarker. However, bacterial symbiont sterol 24-C-methyltransferases is capable of the alkylation of desmosterol to other sponge biomarkers, 24-isopropenylcholesterol, and 24-isopropylidenecholesterol.[15] There is insignificant data which shows that desmosterol has good preservation potential.
Use as a Biomarker
As a biomarker, desmosterol is important for the Chaetoceros calcitrans[16] and R. setigera and N. closterium[17] diatoms as well as Rhodophyceae.[18] However, desmosterol has the potential to be used as a taxonomic marker for diatoms where it exists as the dominant sterol, such as in Rhizosolenia.[17]
Balsfjorden, Northern Norway
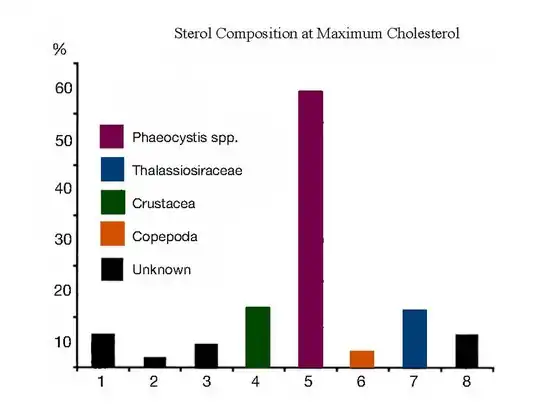
Desmosterol has been used as a biomarker for the presence of copepods. The phytoplankton bloom of P. pouchetii and Thalassiosira decipiens resulted in low amounts of desmosterol at maximum chlorophyll levels, indicating the low abundance and efficient removal of copepods in these phytoplankton.[19]
Peruvian Shelf
Diatoms and Silicoflagellates are classes of phytoplankton present in sedimentary material from the sediment water interface in the Peruvian Shelf region.[20] Mineralogical analysis of this sedimentary material suggested large volumes of phytoplankton. Researchers at the University of Bristol examined the sterol composition of these sedimentary rocks which have gone through oxygen depletion, leading to anoxic conditions. These conditions have enhanced the preservation of lipids present during the time of formation, around 1 year prior, and have limited the degradation of these compounds.
Measurement techniques
Desmosterol has seen to be identified most commonly through chemical constants, ozonolysis, infrared spectroscopy, NMR, and mass spectrometry techniques.[10] The use of thin-layer and gas-liquid chromatography to measure desmosterol is less common with the development of more advanced techniques.
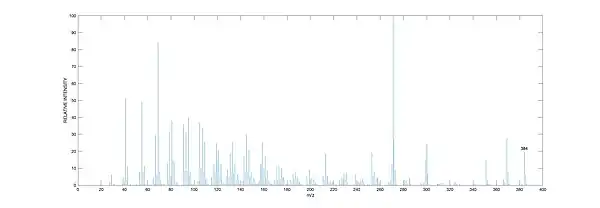
GC-MS
Samples from water sources are first extracted and partially purified before analysis. Sterols are commonly first derivatised after purification. Lipid extracts can be separated into their fragments by ionisation using Gas Chromatography followed by Mass Spectroscopy analysis. Desmosterol has a characteristic m/z peak at 384, as seen in the mass spectra.[6]
Ozonolysis
Desmosterol has been identified through ozonolysis of the sample, with the ozonolysis product of desmosterol having a melting point of 128 °C, and an Rf value of 0.31 with paper-chromatography with n-hexane and N,N-dimethyl formamide. The ozonolysis product also has ratios C: 45.68; H: 4.45; N: 23.21.[10]
See also
References
- ↑ https://www.sciencedirect.com/science/article/pii/S0927775723005733
- ↑ Bloch, Konrad (October 1965). "The Biological Synthesis of Cholesterol". Science. 150 (3692): 19–28. Bibcode:1965Sci...150...19B. doi:10.1126/science.150.3692.19. ISSN 0036-8075. PMID 5319508.
- ↑ Keber, R.; Rozman, D.; Horvat, S. (23 October 2012). "Sterols in spermatogenesis and sperm maturation". The Journal of Lipid Research. 54 (1): 20–33. doi:10.1194/jlr.R032326. PMC 3520525. PMID 23093550. Retrieved 30 June 2015.
- ↑ "Announcing ISMCBBPR's Molecule of the Year 2012". Archived from the original on 2015-09-24. Retrieved 2014-02-22.
- 1 2 Smith, D.J.; Eglinton, G.; Morris, R.J.; Poutanen, E.L. (1983). "Aspects of the steroid geochemistry of an interfacial sediment from the Peruvian upwelling". Oceanologica Acta. 6 (2): 211–219.
- 1 2 Belt, Simon T.; Brown, Thomas A.; Smik, Lukas; Tatarek, Agnieszka; Wiktor, Józef; Stowasser, Gabriele; Assmy, Philipp; Allen, Claire S.; Husum, Katrine (2017-08-01). "Identification of C25 highly branched isoprenoid (HBI) alkenes in diatoms of the genus Rhizosolenia in polar and sub-polar marine phytoplankton". Organic Geochemistry. 110: 65–72. Bibcode:2017OrGeo.110...65B. doi:10.1016/j.orggeochem.2017.05.007. hdl:10026.1/9642. ISSN 0146-6380.
- ↑ Stokes, W. M.; Fish, W. A.; Hickey, F. C. (May 1956). "Metabolism of cholesterol in the chick embryo. II. Isolation and chemical nature of two companion sterols". The Journal of Biological Chemistry. 220 (1): 415–430. doi:10.1016/S0021-9258(18)65366-9. ISSN 0021-9258. PMID 13319360.
- 1 2 Fagerlund, U. H. M.; Idler, D. R. (December 1957). "Marine Sterols. IV. 24-Dehydrocholesterol: Isolation from a Barnacle and Synthesis by the Wittig Reaction". Journal of the American Chemical Society. 79 (24): 6473–6475. doi:10.1021/ja01581a030. ISSN 0002-7863.
- ↑ Gagosian, Robert (1974). Summary of Investigations Conducted in 1974. Massachusetts: Woods Hole Oceanographic Institution. pp. C-16.
- 1 2 3 4 5 6 Idler, D; Saito, A; Wiseman, P (April 1968). "Sterols in red algae (Rhodophyceae)1". Steroids. 11 (4): 465–473. doi:10.1016/S0039-128X(68)80062-5. PMID 5643148.
- ↑ Kobayashi, Masaru; Nishizawa, Motohito; Todo, Kagemi; Mitsuhashi, Hiroshi (1973). "Marine Sterols. I. Sterols of Annelida, Pseudopotamilla occelata MOORE". Chemical & Pharmaceutical Bulletin. 21 (2): 323–328. doi:10.1248/cpb.21.323.
- ↑ Idler, D.R.; Wiseman, P. (October 1971). "Sterols of molluscs". International Journal of Biochemistry. 2 (11): 516–528. doi:10.1016/0020-711X(71)90021-8.
- ↑ Singh, Pushpendra; Saxena, Roopali; Srinivas, Gunda; Pande, Gopal; Chattopadhyay, Amitabha (2013-03-15). Koval, Michael (ed.). "Cholesterol Biosynthesis and Homeostasis in Regulation of the Cell Cycle". PLOS ONE. 8 (3): e58833. Bibcode:2013PLoSO...858833S. doi:10.1371/journal.pone.0058833. ISSN 1932-6203. PMC 3598952. PMID 23554937.
- ↑ "The Nobel Prize in Physiology or Medicine 1964". NobelPrize.org. Retrieved 2023-05-21.
- ↑ Brown, Malory O.; Olagunju, Babatunde O.; Giner, José-Luis; Welander, Paula V. (2022-05-16). "Bacterial sterol methylation confounds eukaryotic biomarker interpretations". doi:10.1101/2022.05.16.491679. S2CID 252914642.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Comp. biochem. physiol". Comparative Biochemistry and Physiology. 9 (2): 160. June 1963. doi:10.1016/0010-406x(63)90020-3. ISSN 0010-406X.
- 1 2 Barrett, Stephanie M.; Volkman, John K.; Dunstan, Graeme A.; LeRoi, Jeannie-Marie (June 1995). "Sterols of 14 Species of Marine Diatoms (Bacillariophyta)1". Journal of Phycology. 31 (3): 360–369. doi:10.1111/j.0022-3646.1995.00360.x. ISSN 0022-3646. S2CID 84292971.
- ↑ "Handbook of Psychopharmacology. Edited by L. L. Iversen, S. D. Iversen and S. H. Snyder. Plenum Press: New York. 1978. - Volume 7Principles of Behavioral Pharmacology. (Pp. 453; illustrated; $34.50.) Plenum Press: New York. 1978. - Volume 8Drugs, Neurotransmitters, and Behavior. (Pp. 590; illustrated; $39.50.) Plenum Press: New York. 1978. - Volume 9Chemical Pathways in the Brain. (Pp. 410; illustrated; $29.50.) Plenum Press: New York. 1978. - Volume 10Neuroleptics and Schizophrenia. (Pp. 250; illustrated; $25.00.) Plenum Press: New York. 1978. - Volume 11Stimulants. (Pp. 476; illustrated; $32.50.) Plenum Press: New York. 1978. - Volume 12Drugs of Abuse. (Pp. 420; illustrated; $32.50.) Plenum Press: New York. 1978. - Volume 13Biology of Mood and Anti-anxiety Drugs. (Pp. 440; illustrated; $32.50.) Plenum Press: New York. 1978. - Volume 14Affective Disorders: Drug Actions in Animals and Man. (Pp. 379; illustrated; $29.50.) Plenum Press: New York. 1978". Psychological Medicine. 9 (1): 194–195. February 1979. doi:10.1017/s0033291700021735. ISSN 0033-2917.
- ↑ Hamm, Christian; Reigstad, Marit; Riser, Christian Wexels; Mühlebach, Anneke; Wassmann, Paul (2001). "On the trophic fate of Phaeocystis pouchetii.: VII. Sterols and fatty acids reveal sedimentation of P. pouchetii-derived organic matter via krill fecal strings". Marine Ecology Progress Series. 209: 55–69. doi:10.3354/meps209055. ISSN 0171-8630. JSTOR 24863836.
- ↑ Smith, D.J.; Eglinton, G.; Morris, R.J.; Poutanen, E.L. (1983). "Aspects of the steroid geochemistry of an interfacial sediment from the Peruvian upwelling" (PDF). Oceanologicaacta. 6 (2): 211–219 – via Ifremer.
- ↑ "Desmosterol". webbook.nist.gov. 17 October 2019. Retrieved 2023-06-10.