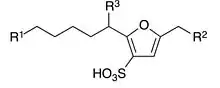
General chemical structure of drilodefensins
Drilodefensins are a class of molecules first found in the gut of earthworms. They belong to the class of dialkylfuransulfonic acids and so far six different homologs have been found.[1]
Origin
They were named based on megadrile, the group of terrestrial clitellate annelids including earthworms. The major occurring drilodefensin in earthworms is drilodefensin 1 (hexylethylfuransulfonic acid, former abbreviated as HEFS[2]).
Mechanism of action
Based on their structure they act as biological surfactants involved in earthworm digestive functioning and protect the earthworm digestive system from the negative impact of plant compounds called polyphenols which do protein-binding.[1]
References
- 1 2 Liebeke, Manuel; Strittmatter, Nicole; Fearn, Sarah; Morgan, A. John; Kille, Peter; Fuchser, Jens; Wallis, David; Palchykov, Vitalii; Robertson, Jeremy (2015-08-04). "Unique metabolites protect earthworms against plant polyphenols". Nature Communications. 6: 7869. doi:10.1038/ncomms8869. PMC 4532835. PMID 26241769.
- ↑ Bundy, Jacob G.; Lenz, Eva M.; Bailey, Nigel J.; Gavaghan, Claire L.; Svendsen, Claus; Spurgeon, David; Hankard, Peter K.; Osborn, Daniel; Weeks, Jason M. (2002-09-01). "Metabonomic assessment of toxicity of 4-fluoroaniline, 3,5-difluoroaniline and 2-fluoro-4-methylaniline to the earthworm Eisenia veneta (rosa): Identification of new endogenous biomarkers". Environmental Toxicology and Chemistry. 21 (9): 1966–1972. doi:10.1002/etc.5620210926. ISSN 1552-8618.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.