Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the EIF5A gene.[5]
It is the only known protein to contain the unusual amino acid hypusine [Nε-(4-amino-2-hydroxybutyl)-lysine], which is synthesized on eIF5A at a specific lysine residue from the polyamine spermidine by two catalytic steps.[6]
EF-P is the bacterial homolog of eIF5A, which is modified post-translationally in a similar but distinct way.[7][8] Both proteins are believed to catalyze peptide bond formation and help resolve ribosomal stalls, making them elongation factors despite the "initiation factor" name originally assigned.[9]
Clinical relevance
Germline deleterious heterozygous EIF5A variants cause Faundes-Banka syndrome.[10][11] This rare human disorder is characterized by variable combinations of developmental delay, microcephaly, micrognathia and dysmorphic features.
References
- 1 2 3 ENSG00000288145 GRCh38: Ensembl release 89: ENSG00000132507, ENSG00000288145 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000078812 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
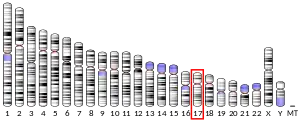
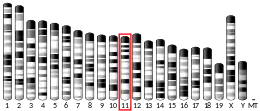
- ↑ Steinkasserer A, Jones T, Sheer D, Koettnitz K, Hauber J, Bevec D (February 1995). "The eukaryotic cofactor for the human immunodeficiency virus type 1 (HIV-1) rev protein, eIF-5A, maps to chromosome 17p12-p13: three eIF-5A pseudogenes map to 10q23.3, 17q25, and 19q13.2". Genomics. 25 (3): 749–752. doi:10.1016/0888-7543(95)80025-H. PMID 7759117.
- ↑ Wolff EC, Kang KR, Kim YS, Park MH (August 2007). "Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification". Amino Acids. 33 (2): 341–350. doi:10.1007/s00726-007-0525-0. PMC 2572820. PMID 17476569.
- ↑ Park JH, Johansson HE, Aoki H, Huang BX, Kim HY, Ganoza MC, Park MH (January 2012). "Post-translational modification by β-lysylation is required for activity of Escherichia coli elongation factor P (EF-P)". The Journal of Biological Chemistry. 287 (4): 2579–2590. doi:10.1074/jbc.M111.309633. PMC 3268417. PMID 22128152.
- ↑ Peil L, Starosta AL, Virumäe K, Atkinson GC, Tenson T, Remme J, Wilson DN (August 2012). "Lys34 of translation elongation factor EF-P is hydroxylated by YfcM". Nature Chemical Biology. 8 (8): 695–697. doi:10.1038/nchembio.1001. PMID 22706199.
- ↑ Rossi D, Kuroshu R, Zanelli CF, Valentini SR (2013). "eIF5A and EF-P: two unique translation factors are now traveling the same road". Wiley Interdisciplinary Reviews. RNA. 5 (2): 209–222. doi:10.1002/wrna.1211. PMID 24402910. S2CID 25447826.
- ↑ Faundes V, Jennings MD, Crilly S, Legraie S, Withers SE, Cuvertino S, Davies SJ, Douglas AG, Fry AE, Harrison V, Amiel J, Lehalle D, Newman WG, Newkirk P, Ranells J, Splitt M, Cross LA, Saunders CJ, Sullivan BR, Granadillo JL, Gordon CT, Kasher PR, Pavitt GD, Banka S (February 2021). "Impaired eIF5A function causes a Mendelian disorder that is partially rescued in model systems by spermidine". Nature Communications. 12 (1): 833. Bibcode:2021NatCo..12..833F. doi:10.1038/s41467-021-21053-2. PMC 7864902. PMID 33547280.
- ↑ "OMIM Entry - # 619376 - FAUNDES-BANKA SYNDROME; FABAS". omim.org. Retrieved 2022-01-03.
Further reading
- Bevec D, Hauber J (1997). "Eukaryotic initiation factor 5A activity and HIV-1 Rev function". Biological Signals. 6 (3): 124–133. doi:10.1159/000109118. PMID 9285095.
- Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY (2006). "Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions". Cell Research. 15 (11–12): 923–934. doi:10.1038/sj.cr.7290370. PMID 16354571.
- Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (December 1992). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis. 13 (12): 960–969. doi:10.1002/elps.11501301199. PMID 1286667. S2CID 41855774.
- Chung SI, Park MH, Folk JE, Lewis MS (February 1991). "Eukaryotic initiation factor 5A: the molecular form of the hypusine-containing protein from human erythrocytes". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1076 (3): 448–451. doi:10.1016/0167-4838(91)90490-q. PMID 1900436.
- Smit-McBride Z, Dever TE, Hershey JW, Merrick WC (January 1989). "Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein". The Journal of Biological Chemistry. 264 (3): 1578–1583. doi:10.1016/S0021-9258(18)94226-2. PMID 2492279.
- Park MH, Liu TY, Neece SH, Swiggard WJ (November 1986). "Eukaryotic initiation factor 4D. Purification from human red blood cells and the sequence of amino acids around its single hypusine residue". The Journal of Biological Chemistry. 261 (31): 14515–14519. doi:10.1016/S0021-9258(18)66899-1. PMID 3095320.
- Koettnitz K, Kappel B, Baumruker T, Hauber J, Bevec D (July 1994). "The genomic structure encoding human initiation factor eIF-5A". Gene. 144 (2): 249–252. doi:10.1016/0378-1119(94)90385-9. PMID 7545941.
- Klier H, Csonga R, Joäo HC, Eckerskorn C, Auer M, Lottspeich F, Eder J (November 1995). "Isolation and structural characterization of different isoforms of the hypusine-containing protein eIF-5A from HeLa cells". Biochemistry. 34 (45): 14693–14702. doi:10.1021/bi00045a010. PMID 7578077.
- Koettnitz K, Wöhl T, Kappel B, Lottspeich F, Hauber J, Bevec D (July 1995). "Identification of a new member of the human eIF-5A gene family". Gene. 159 (2): 283–284. doi:10.1016/0378-1119(95)00136-T. PMID 7622067.
- Joe YA, Park MH (October 1994). "Structural features of the eIF-5A precursor required for posttranslational synthesis of deoxyhypusine". The Journal of Biological Chemistry. 269 (41): 25916–25921. doi:10.1016/S0021-9258(18)47333-4. PMID 7929297.
- Bevec D, Klier H, Holter W, Tschachler E, Valent P, Lottspeich F, et al. (November 1994). "Induced gene expression of the hypusine-containing protein eukaryotic initiation factor 5A in activated human T lymphocytes". Proceedings of the National Academy of Sciences of the United States of America. 91 (23): 10829–10833. Bibcode:1994PNAS...9110829B. doi:10.1073/pnas.91.23.10829. PMC 45119. PMID 7971969.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–174. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Ruhl M, Himmelspach M, Bahr GM, Hammerschmid F, Jaksche H, Wolff B, et al. (December 1993). "Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation". The Journal of Cell Biology. 123 (6 Pt 1): 1309–1320. doi:10.1083/jcb.123.6.1309. PMC 2290910. PMID 8253832.
- Liu YP, Nemeroff M, Yan YP, Chen KY (1997). "Interaction of eukaryotic initiation factor 5A with the human immunodeficiency virus type 1 Rev response element RNA and U6 snRNA requires deoxyhypusine or hypusine modification". Biological Signals. 6 (3): 166–174. doi:10.1159/000109123. PMID 9285100.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–156. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Singh US, Li Q, Cerione R (January 1998). "Identification of the eukaryotic initiation factor 5A as a retinoic acid-stimulated cellular binding partner for tissue transglutaminase II". The Journal of Biological Chemistry. 273 (4): 1946–1950. doi:10.1074/jbc.273.4.1946. PMID 9442029.
- Schatz O, Oft M, Dascher C, Schebesta M, Rosorius O, Jaksche H, et al. (February 1998). "Interaction of the HIV-1 rev cofactor eukaryotic initiation factor 5A with ribosomal protein L5". Proceedings of the National Academy of Sciences of the United States of America. 95 (4): 1607–1612. Bibcode:1998PNAS...95.1607S. doi:10.1073/pnas.95.4.1607. PMC 19115. PMID 9465063.
- Lee YB, Joe YA, Wolff EC, Dimitriadis EK, Park MH (May 1999). "Complex formation between deoxyhypusine synthase and its protein substrate, the eukaryotic translation initiation factor 5A (eIF5A) precursor". The Biochemical Journal. 340 (1): 273–281. doi:10.1042/0264-6021:3400273. PMC 1220246. PMID 10229683.