A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives.[1] Most aromatic polyamines are crystalline solids at room temperature.
Natural polyamines
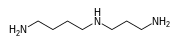
Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC).[2] Polyamines are found in high concentrations in the mammalian brain.[3]
- Natural polyamines

Synthetic polyamines
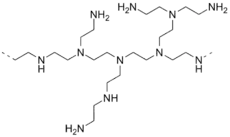
Ethyleneamines are a commercially-important class of synthetic polyamines with ethylene (-CH2CH2- linkages); global production capacity was estimated at 385,000 tonnes in 2001.[4] They are chemical intermediates often used to make surfactants and as crosslinkers for epoxy resins.[5] Some interesting members of this class include:
- Ethylenediamine, first member of this series. It is a chelating ligand by itself, and it is a precursor to the popular metal sequestrant, EDTA (ethylenediaminetetraacetic acid). Permethylated, ethylenediamine yields tetramethylethylenediamine (TMEDA) that has a very high affinity for lithium ions.[6]
- Macrocyclic polyamines analogous to crown ethers: 1,4,7-triazacyclononane ((NHCH2CH2)3) and cyclen ((NHCH2CH2)4). A related tetraaza macrocycle is cyclam.
- Tris(2-aminoethyl)amine (N(CH2CH2NH2)3) is a branched polyamine that is a minor side product of the polyethyleneamine process. A related tripodal polyamine is 1,1,1-tris(aminomethyl)ethane. These are interesting chelating ligands.
- Polyethylenimine is a polymer derived from aziridine.
Other synthetic polyamines include 1,3,5-triazinane (not to be confused with 1,3,5-triazine) and N-substituted analogs. The methylene (-CH2) linkages are derived from formaldehyde. The reaction product of monoethanolamine and formaldehyde is known industrially as "MEA triazine" (it is actually a triazinane), and it serves as a water-soluble hydrogen sulfide scavenger.[7] Hexamethylenetetramine (hexamine) is another product of formaldehyde and ammonia that has various uses in industry. Domestically, it is used as a solid camping fuel. In the laboratory, it reacts with alkyl halides to selectively prepare primary amines in the Delépine reaction.
- Synthetic polyamines
amine.svg.png.webp)


ethane.svg.png.webp)
 Subunit of polyethylenimine
Subunit of polyethylenimine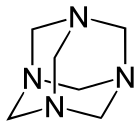 Hexamethylenetetramine with its adamantane-type structure
Hexamethylenetetramine with its adamantane-type structure
Biological function
Although it is known that the biosynthesis of polyamines is highly regulated, the biological function of polyamines is only partly understood. In their cationic ammonium form, they bind to DNA, and, in structure, they represent compounds with cations that are found at regularly spaced intervals (in contrast to Mg2+
or Ca2+
, which are point charges). They have also been found to act as promoters of programmed ribosomal frameshifting during translation.[8]
Inhibition of polyamine biosynthesis retards or stops cell growth. The provision of exogenous polyamines restores the growth of these cells. Most eukaryotic cells express a polyamine-transporting ATPase on their cell membrane that facilitates the internalization of exogenous polyamines. This system is highly active in rapidly proliferating cells and is the target of some chemotherapeutics currently under development.[9]
Polyamines are also modulators of a variety of ion channels, including NMDA receptors and AMPA receptors. They block inward-rectifier potassium channels so that the currents of the channels are inwardly rectified, thereby the cellular energy, i.e. K+
ion gradient across the cell membrane, is conserved. In addition, polyamine participate in initiating the expression of SOS response of Colicin E7 operon and down-regulate proteins that are essential for colicin E7 uptake, thus conferring a survival advantage on colicin-producing E. coli under stress conditions.[10]
Polyamines can enhance the permeability of the blood–brain barrier.[11]
They are involved in modulating senescence of organs in plants and are therefore considered as a plant hormone.[12] In addition, they are directly involved in regulation of programmed cell death.[13]
Homology-directed DNA repair
Polyamines promote homologous recombination (HR)-mediated double-strand break (DSB) repair.[14] Polyamines enhance the DNA strand exchange activity of RAD51 recombinase. Depletion of polyamines sensitizes cells to genotoxic substances such as ionizing radiation and ultraviolet radiation. The effect of polyamines on RAD51 arises from their ability to enhance the capture of homologous duplex DNA and promote RAD-51-mediated homologous DNA pairing and exchange activity.[14] Polyamines appear to have an evolutionarily conserved role in regulating recombinase activity.
Biosynthesis of spermidine, spermine, thermospermine

Spermidine is synthesized from putrescine, using an aminopropyl group from decarboxylated S-adenosyl-L-methionine (SAM), S-Adenosylmethioninamine. The reaction is catalyzed by spermidine synthase.[15]
Spermine is synthesized from the reaction of spermidine with SAM in the presence of the enzyme spermine synthase.
The polyamines undergo rapid interconversion in the polyamine cycle, in which putrescine leads to synthesis of spermidine and spermine, with degradation of these polyamines to form putrescine, which can begin the cycle again.[15]
Thermospermine (NH2-(CH2)3-NH-(CH2)3-NH-(CH2)4-NH2) is a structural isomer of spermine and a novel type of plant growth regulator. It is produced from spermidine by the action of thermospermine synthase, which is encoded by a gene named ACAULIS5 (ACL5).[16]
Polyamine analogues
The critical role of polyamines in cell growth has led to the development of a number of agents that interfere with polyamine metabolism. These agents are used in cancer therapy. Polyamine analogues upregulate p53 in a cell leading to restriction of proliferation and apoptosis.[17] It also decreases the expression of estrogen receptor alpha in ER-positive breast cancer.[18]
See also
References
- ↑ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 978-3527306732.
- ↑ Pegg, AE; McCann, PP (1982). "Polyamine metabolism and function". American Journal of Physiology. 243 (5): 212–21. doi:10.1152/ajpcell.1982.243.5.C212. PMID 6814260. S2CID 21063248.
- ↑ Seiler, N (1992). "Polyamines". Handbook of Neurochemistry. Vol. 1. New York, NY: Plenum Publishing Corp. pp. 223–55.
- ↑ Srivasan Sridhar; Richard G. Carter (2001). "Diamines and Higher Amines, Aliphatic". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley. doi:10.1002/0471238961.0409011303011820.a01.pub2. ISBN 9780471238966.
- ↑ Lawrence, Stephen A. (2004). Amines: synthesis, properties and applications. Cambridge University Press. p. 64. ISBN 978-0-521-78284-5.
- ↑ Haynes, R. K.; Vonwiller, S. C.; Luderer, M. R. (2006). "N,N,N′,N′-Tetramethylethylenediamine". In Paquette, L. (ed.). N,N,N′,N′-Tetramethylethylenediamine. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rt064.pub2. ISBN 0471936235.
- ↑ G. N. Taylor; J. J. Wylde; T. Müller; J Murison; F. Schneider (2017). Fresh Insight into the H2S Scavenging Mechanism of MEA-Triazine vs. MMA-Triazine. SPE International Conference on Oilfield Chemistry. Montgomery, Texas. doi:10.2118/184529-MS.
- ↑ Rato C; Amirova S.R; Bates D.G; Stansfield I; Wallace H.M (June 2011). "Translational recoding as a feedback controller: systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift". Nucleic Acids Res. 39 (11): 4587–4597. doi:10.1093/nar/gkq1349. PMC 3113565. PMID 21303766.
- ↑ Wang C, Delcros JG, Cannon L, et al. (November 2003). "Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters". J. Med. Chem. 46 (24): 5129–38. doi:10.1021/jm030223a. PMID 14613316.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ Yi-Hsuan Pan; Chen-Chung Liao (May 2006). "The critical roles of polyamines regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli". J. Biol. Chem. 281 (19): 13083–13091. doi:10.1074/jbc.M511365200. PMID 16549429.
- ↑ Zhang L, Lee HK, Pruess TH, White HS, Bulaj G (March 2009). "Synthesis and applications of polyamine amino acid residues: improving the bioactivity of an analgesic neuropeptide, neurotensin". J. Med. Chem. 52 (6): 1514–7. doi:10.1021/jm801481y. PMC 2694617. PMID 19236044.
- ↑ Pandey S, Ranade SA, Nagar PK, Kumar N (September 2000). "Role of polyamines and ethylene as modulators of plant senescence". J. Biosci. 25 (3): 291–9. doi:10.1007/BF02703938. PMID 11022232. S2CID 21925829.
- ↑ Moschou, PN; Roubelakis-Angelakis, KA (Nov 11, 2013). "Polyamines and programmed cell death". Journal of Experimental Botany. 65 (5): 1285–1296. doi:10.1093/jxb/ert373. PMID 24218329.
- 1 2 Lee CY, Su GC, Huang WY, Ko MY, Yeh HY, Chang GD, Lin SJ, Chi P. Promotion of homology-directed DNA repair by polyamines. Nat Commun. 2019 Jan 8;10(1):65. doi: 10.1038/s41467-018-08011-1. PMID 30622262; PMCID: PMC6325121
- 1 2 Pál M, Szalai G, Janda T (2015). "Speculation: Polyamines are important in abiotic stress signaling" (PDF). Plant Science. 237: 16–23. doi:10.1016/j.plantsci.2015.05.003. PMID 26089148.
- ↑ Takano, A; Kakehi, J; Takahashi, T (April 2012). "Thermospermine is not a minor polyamine in the plant kingdom". Plant Cell Physiol. 53 (4): 606–16. doi:10.1093/pcp/pcs019. PMID 22366038.
- ↑ Huang, Yi; Pledgie, Allison; Rubin, Ethel; Marton, Laurence J.; Woster, Patrick M.; Sukumar, Saraswati; Casero, Robert A.; Davidson, Nancy E. (September 2005). "Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells". Cancer Biology & Therapy. 4 (9): 1006–1013. doi:10.4161/cbt.4.9.1970. PMC 3639297. PMID 16131835.
- ↑ Huang, Y; Keen, JC; Pledgie, A; Marton, LJ; Zhu, T; Sukumar, S; Park, BH; Blair, B; Brenner, K; Casero, RA Jr; Davidson, NE (2006). "Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells". J Biol Chem. 281 (28): 19055–63. doi:10.1074/jbc.M600910200. PMC 3623667. PMID 16679312.
External links
- Polyamines in cell cycle proliferation and cell death
- Ornithine Decarboxylase: Expression and regulation in rat brain and in transgenic mice, 2002, Pekka Kilpelainen, Department of Biochemistry, University of Oulu. Extensive review of literature through 2001 on polyamine structure, properties, metabolism in mammals, and physiological and pathophysiological roles (See article Table of Contents)