 | |
| Names | |
|---|---|
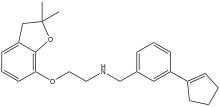
| Preferred IUPAC name
N-{[3-(Cyclopent-1-en-1-yl)phenyl]methyl}-2-[(2,2-dimethyl-2,3-dihydro-1-benzofuran-7-yl)oxy]ethan-1-amine | |
| Other names
F-15,063 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C24H29NO2 | |
| Molar mass | 363.49 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
F-15,063 is an orally active potential antipsychotic, and an antagonist at the D2/D3 receptors, partial agonist at the D4 receptor, and agonist at the 5-HT1A receptors. It has greater efficacy at the 5-HT1A receptors than other antipsychotics, such as clozapine, aripiprazole, and ziprasidone. This greater efficacy may lead to enhanced antipsychotic properties, as antipsychotics that lack 5-HT1A affinity are associated with increased risk of extrapyramidal symptoms, and lack of activity against the negative symptoms of schizophrenia.[1]
As expression of immediate-early gene (IEG) in certain brain regions may represent markers of anti-psychotic activity, expression of immediate-early gene mRNA in the prefrontal cortex and striatum was measured. Treatment with F-15,063 resulted in induction of c-fos and fosB mRNA expression in the prefrontal cortex. In the striatum, F-15,063 treatment resulted in induction of all IEGs studied (c-fos, fosB, zif268, c-jun, junB, nor1, nur77, arc).[2]
F-15,063 was tested in several animal models that predict antipsychotic activity to help determine the behavioral profile. Administration of F-15,063 blocked amphetamine and ketamine induced hyperlocomotion, but did not affect baseline, spontaneous locomotor activity. In addition, F-15,063 did not produce catalepsy, a side effect of other antipsychotics, such as haloperidol. This inhibition of catalepsy is 5-HT1A receptor mediated, as pretreatment with the 5-HT1A antagonist WAY-100635 reinstated catalepsy. The level of catalepsy did not increase with chronic dosing, and there was no evidence for serotonin syndrome at clinically relevant doses.[3]
Plasma levels of F-15,063 decreased seven-fold 4 hours after oral administration, and 25-fold after 8 hours. Despite this, there was still a high (65%) level of central D2 occupancy at 4 hours, and it retained its full antipsychotic potential at this time point. Even after 8 hours, F-15,063 still demonstrated some central D2 occupancy, and retained some antipsychotic activity.[4]
References
- ↑ Newman-Tancredi, A. (May 2007). "F15063, a potential antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (I) in vitro receptor affinity and efficacy profile". British Journal of Pharmacology. 151 (2): 237–52. doi:10.1038/sj.bjp.0707158. PMC 2013955. PMID 17375087.
- ↑ Bruins Slot, LA (October 2009). "F15063, a potential antipsychotic with dopamine D(2)/D(3) receptor antagonist and 5-HT(1A) receptor agonist properties: influence on immediate-early gene expression in rat prefrontal cortex and striatum". European Journal of Pharmacology. 620 (1–3): 27–35. doi:10.1016/j.ejphar.2009.08.019. PMID 19695244.
- ↑ Depoortère, R. (May 2007). "F15063, a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. II. Activity in models of positive symptoms of schizophrenia". British Journal of Pharmacology. 151 (2): 253–65. doi:10.1038/sj.bjp.0707159. PMC 2013947. PMID 17375086.
- ↑ Assié, MB (June 2007). "F15063, a potential antipsychotic with dopamine D(2)/D(3) antagonist, 5-HT(1A) agonist and D(4) partial agonist properties: (IV) duration of brain D2-like receptor occupancy and antipsychotic-like activity versus plasma concentration in mice". Naunyn-Schmiedeberg's Archives of Pharmacology. 375 (4): 241–50. doi:10.1007/s00210-007-0162-x. PMID 17453175. S2CID 20594732.