| Monofunctional compounds |
|---|
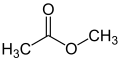 |
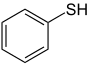 |
| Difunctional compounds |
|---|
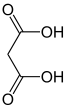 |
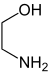 |
 |
| Trifunctional compounds |
|---|
 |
-Cysteine_Structural_Formula_V1.svg.png.webp) |
In chemistry, functionality is the presence of functional groups in a molecule. A monofunctional molecule possesses one functional group, a bifunctional (or difunctional) two, a trifunctional three, and so forth. In organic chemistry (and other fields of chemistry), a molecule's functionality has a decisive influence on its reactivity.
In polymer chemistry, the functionality of a monomer refers to its number of polymerizable groups, and affects the formation and the degree of crosslinking of polymers.
In organic chemistry and material science
In organic chemistry, functionality is often used as a synonym for functional group. For example, a hydroxyl group can also be called a HO-function.[1][2]
Functionalisation means the introduction of functional groups, for example
- the functionalisation of a surface[3] (e.g. silanization for the specific modification of the adhesion of a surface)
- the functionalization of nanoparticles of a metal or metal oxide to stabilize such nanoparticles[4] or
- the so-called C-H functionalization,[5] which means the substitution of a C-H bond by a functional group, bonded at the same carbon atom
In polymer chemistry
According to IUPAC, the functionality of a monomer is defined as the number of bonds that a monomer's repeating unit forms in a polymer with other monomers. Thus in the case of a functionality of f = 2 a linear polymer is formed by polymerizing (a thermoplastic). Monomers with a functionality f ≥ 3 lead to a branching point, which can lead to cross-linked polymers (a thermosetting polymer). Monofunctional monomers do not exist as such molecules lead to a chain termination.[6]
From the average functionality of the used monomers the reaching of the gel point can be calculated as a function of reaction progress.[7] Side reactions may increase or decrease the functionality.[8]
However, IUPAC definition and the use of the term in organic chemistry differ with respect to the functionality of a double bond.[6][9] In polymer chemistry, a double bond possesses a functionality of two (because two points of contact for further polymer chains are present, on each of the two adjacent carbon atoms), while in organic chemistry the double bond is a functional group and thus has a functionality of one.
See also
References
- ↑ Kurt Peter C. Vollhardt, Neil Eric Schore: Organische Chemie, S. 73 (, p. 74, at Google Books).
- ↑ Riedel: Moderne Anorganische Chemie von Christoph Janiak, S. 401 (, p. 401, at Google Books).
- ↑ Alexander Langner, Anthony Panarello, Sandrine Rivillon, Oleksiy Vassylyev, Johannes G. Khinast, Yves J. Chabal: Controlled Silicon Surface Functionalization by Alkene Hydrosilylation, J. Am. Chem.
- ↑ Marie-Alexandra Neouze, Ulrich Schubert: Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands, Monatsh.
- ↑ Dirk Steinborn: Grundlagen der metallorganischen Komplexkatalyse, S. 305 (, p. 239, at Google Books
- 1 2 Eintrag zu functionality, f of a monomer.
- ↑ Koltzenburg: Polymere: Synthese, Eigenschaften und Anwendungen, S. 187 (, p. 188, at Google Books). This reference is being translated to English as "Polymer Chemistry" by the same authors, to appear in September 2017. See
- ↑ Hans-Georg Elias: Makromoleküle: Chemische Struktur und Synthesen, S. 468 und 477 (, p. 468, at Google Books).
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chemical functionality". doi:10.1351/goldbook.CT07503