 | |
| Names | |
|---|---|
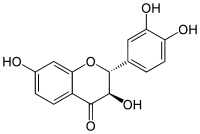
| IUPAC name
(2R,3R)-3,3′,4′,7-Tetrahydroxyflavan-4-one | |
| Systematic IUPAC name
(2R,3R)-2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
2,3-Dihydrofisetin 3,7,3',4'-Tetrahydroxyflavanone 2,3-Dihydrofisetin 3′,4′,7-Trihydroxyflavanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.039.975 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H12O6 | |
| Molar mass | 288.255 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Fustin, sometimes called "dihydrofisetin", is a flavanonol, a type of flavonoid. It can be found in young fustic (Cotinus coggygria)[1] and in the lacquer tree (Toxicodendron vernicifluum).[2]
Fustin shows protective effects on 6-hydroxydopamine-induced neuronal cell death.[2]
Unlike fisetin, fustin has no double bond in the C-ring. This makes fustin a flavan, with two stereocenters and four stereoisomers.
References
- ↑ Valianou, Lemonia; Stathopoulou, Konstantina; Karapanagiotis, Ioannis; Magiatis, Prokopios; Pavlidou, Eleni; Skaltsounis, Alexios-Leandros; Chryssoulakis, Yannis (2009). "Phytochemical analysis of young fustic (Cotinus coggygria heartwood) and identification of isolated colourants in historical textiles". Analytical and Bioanalytical Chemistry. 394 (3): 871. doi:10.1007/s00216-009-2767-z. PMID 19352635. S2CID 22188491.
- 1 2 Park, Byung Chul; Lee, Yong Soo; Park, Hee-Juhn; Kwak, Mi-Kyoung; Yoo, Bong Kyu; Kim, Joo Young; Kim, Jung-Ae (2007). "Protective effects of fustin, a flavonoid from Rhus verniciflua Stokes, on 6-hydroxydopamine-induced neuronal cell death". Experimental & Molecular Medicine. 39 (3): 316. doi:10.1038/emm.2007.35. PMID 17603285.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.