
The GUS reporter system (GUS: β-glucuronidase) is a reporter gene system, particularly useful in plant molecular biology[1] and microbiology.[2] Several kinds of GUS reporter gene assay are available, depending on the substrate used. The term GUS staining refers to the most common of these, a histochemical technique.
Purpose
The purpose of this technique is to analyze the activity of a gene transcription promoter (in terms of expression of a so-called reporter gene under the regulatory control of that promoter) either in a quantitative manner, involving some measure of activity, or qualitatively (on versus off) through visualization of its activity in different cells, tissues, or organs. The technique utilizes the uidA gene of Escherichia coli, which codes for the enzyme, β-glucuronidase;[3] this enzyme, when incubated with specific colorless or non-fluorescent substrates, can convert them into stable colored or fluorescent products.[4] The presence of the GUS-induced color indicates where the gene has been actively expressed. In this way, strong promoter activity produces much staining and weak promoter activity produces less staining.
The uidA gene can also be fused to a gene of interest, creating a gene fusion. The insertion of the uidA gene will cause production of GUS, which can then be detected using various glucuronides as substrates.[4]
Substrates
There are different possible glucuronide that can be used as substrates for the β-glucuronidase, depending on the type of detection needed (histochemical, spectrophotometrical, fluorimetrical). The most common substrate for GUS histochemical staining is 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc). X-Gluc is hydrolyzed by GUS into the product 5,5'-dibromo-4,4'-dichloro-indigo (diX-indigo). DiX-indigo will appear blue, and can be seen using light microscopy.[5] This process is analogous to hydrolysis of X-gal by Beta-galactosidase[5] to produce blue cells as is commonly practiced in bacterial reporter gene assays.
For other types of detection, common substrates are p-nitrophenyl β-D-glucuronide for the spectrophotometric assay and 4-methylumbelliferyl-beta-D-glucuronide (MUG) for the fluorimetric assay.[6]
History
The system was originally developed by Richard Anthony Jefferson during his Ph.D. at the University of Colorado at Boulder.[7] He adapted the technique for the use with plants as he worked in the Plant Breeding Institute of Cambridge, between 1985 and 1987.[1] Since then thousands of labs have used the system, making it one of the most widely used tools in plant molecular biology, as underlined by thousands of citations in scientific literature.[7]
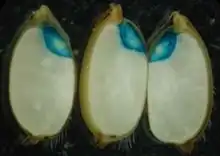
Target organisms
An organism is suitable for a GUS assay if it lacks naturally occurring β-glucuronidase activity or if the activity is very low (background activity). For this reason, the assay is not useful in most vertebrates and many molluscs.[6] Since there is no detectable GUS activity in higher plants, mosses, algae, ferns, fungi and most bacteria,[6] the assay is ideally suited for gene expression studies in these organisms, and considered the reporter gene of choice for in plant science.
Benefits and limitations
The GUS assay does not require the presence of any cofactors or ions for function. Beta-glucuronidase can function through a wide range of pH values, and is fairly resistant to thermal inactivation.[8] However, GUS is susceptible to inhibition from certain heavy metal ions, such as Cu2+ and Zn2+.
Additionally, the interpretation of the assay is limited by the movement of diX-indigo throughout the cell. DiX-indigo, can associate with lipids to diffuse far from the site of enzyme activity, which shows a lack of cytosolic localization and irregularity of substrate penetration. This can potentially lead to an incorrect interpretation of GUS protein localization.[9] Despite a lack of cellular localization, nuclear localization of GUS has been well observed.[10] GUS assays can be carried out in the presence of potassium ferricyanide to prevent the stain from diffusing.[5]
Other reporter systems
The GUS system is not the only available gene reporter system for the analysis of promoter activity. Other competing systems are based on e.g. luciferase, GFP, beta-galactosidase, chloramphenicol acetyltransferase (CAT), alkaline phosphatase. The use of one or the other system is mainly dependent on the organism of interest and the imaging and microscopy technologies available to the laboratories conducting the research.
Other uses

The GUS assay, as well as other reporter gene systems, can be used for other kinds of studies other than the classical promoter activity assay. Reporter systems have been used for the determination of the efficiency of gene delivery systems, the intracellular localization of a gene product, the detection of protein-protein or protein-DNA interactions, the efficiency of translation initiation signals and the success of molecular cloning efforts.
Sources
- 1 2 Jefferson, R. A.; Kavanagh, T. A.; Bevan, M. W. (1987). "GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants". The EMBO Journal. 6 (13): 3901–7. doi:10.1002/j.1460-2075.1987.tb02730.x. PMC 553867. PMID 3327686.
- ↑ Vande Broek, A; Lambrecht, M; Vanderleyden, J (1998). "Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense". Microbiology. 144 (9): 2599–606. doi:10.1099/00221287-144-9-2599. PMID 9782509.
- ↑ Blanco, C; Ritzenthaler, P; Mata-Gilsinger, M (1982). "Cloning and endonuclease restriction analysis of uidA and uidR genes in Escherichia coli K-12: Determination of transcription direction for the uidA gene". Journal of Bacteriology. 149 (2): 587–94. doi:10.1128/JB.149.2.587-594.1982. PMC 216546. PMID 6276362.
- 1 2 Jefferson, R. A.; Burgess, S. M.; Hirsh, D (1986). "Beta-Glucuronidase from Escherichia coli as a gene-fusion marker". Proceedings of the National Academy of Sciences of the United States of America. 83 (22): 8447–51. Bibcode:1986PNAS...83.8447J. doi:10.1073/pnas.83.22.8447. PMC 386947. PMID 3534890.
- 1 2 3 Guivarc'h, A.; Caissard, J. C.; Azmi, A.; Elmayan, T.; Chriqui, D.; Tepfer, M. (1996-09-01). "In situ detection of expression of thegus reporter gene in transgenic plants: ten years of blue genes". Transgenic Research. 5 (5): 281–288. doi:10.1007/BF01968938. ISSN 1573-9368. S2CID 21151079.
- 1 2 3 U.S. Patent 5,268,463
- 1 2 Cambia Organization Website: biography of Richard A. Jefferson Archived 2006-08-19 at the Wayback Machine
- ↑ Jefferson, Richard A. (1987-12-01). "Assaying chimeric genes in plants: The GUS gene fusion system". Plant Molecular Biology Reporter. 5 (4): 387–405. doi:10.1007/BF02667740. ISSN 1572-9818. S2CID 5619830.
- ↑ Caissard, Jean-Claude; Guivarc'h, Anne; Rembur, Jacques; Azmi, Abdelkrim; Chriqui, Dominique (1994-05-01). "Spurious localizations of diX-indigo microcrystals generated by the histochemical GUS assay". Transgenic Research. 3 (3): 176–181. doi:10.1007/BF01973985. ISSN 1573-9368. S2CID 797978.
- ↑ Citovsky, V.; Zupan, J.; Warnick, D.; Zambryski, P. (1992-06-26). "Nuclear localization of Agrobacterium VirE2 protein in plant cells". Science. 256 (5065): 1802–1805. Bibcode:1992Sci...256.1802C. doi:10.1126/science.1615325. ISSN 0036-8075. PMID 1615325.