This page provides supplementary chemical data on glycerol.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4729 at 20°C |
| Abbe number | ? |
| Dielectric constant, εr | 42.5 ε0 at 25 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[1] | 63.4 mN/m at 20°C 58.6 mN/m at 90°C 51.9 mN/m at 150°C |
| Viscosity[2] | 1.412 Pa·s at 20°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 291.8 K (18.7 °C), ~99500 Pa |
| Critical point | 850 K (577 °C), 7500 kPa |
| Std enthalpy change of fusion, ΔfusH |
18.28 kJ/mol |
| Std entropy change of fusion, ΔfusS |
62.7 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
91.7 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
201 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
37.87 J/(mol K)[3] |
| Heat capacity, cp | 150. J/(mol K) 6°C - 11°C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–669.6 kJ/mol |
| Standard molar entropy, S |
206.3 J/(mol K)[4] |
| Enthalpy of combustion, ΔcH |
–1654.3 kJ/mol |
| Heat capacity, cp | 221.9 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–577.9 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | 125.5 | 167.2 | 198.0 | 220.1 | 263.0 | 290.0 | |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed.
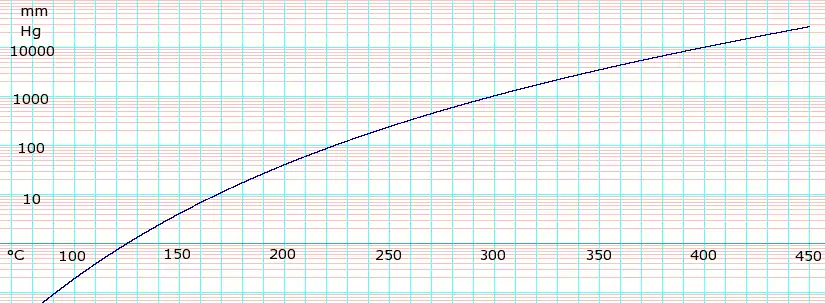
loge of Glycerol vapor pressure. Uses formula: with coefficients A=-2.125867E+01, B=-1.672626E+04, C=1.655099E+02, and D=1.100480E-05 obtained from CHERIC[5]
Freezing point of aqueous solutions
| % glycerol by weight |
10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freezing point °C |
–1.6 | –4.8 | –9.5 | –15.5 | –22.0 | –33.6 | –37.8 | –19.2 | –1.6 | 17.0 | |
| Specific gravity d15° |
1.02415 | 1.04935 | 1.07560 | 1.10255 | 1.12985 | 1.15770 | 1.18540 | 1.21290 | 1.23950 | 1.26557 | |
Table data obtained from Lange's Handbook of Chemistry, 10th ed. Specific gravity is at 15°C, referenced to water at 15°C.
See details on: Freezing Points of Glycerine-Water Solutions Dow Chemical [6] or Freezing Points of Glycerol and Its Aqueous Solutions.[7]
Distillation data
| Vapor-liquid Equilibrium of Glycerol/water[8] P = 760 mmHg | ||
| BP Temp. °C |
% by mole water | |
|---|---|---|
| liquid | vapor | |
| 278.8 | 2.75 | 93.15 |
| 247.0 | 4.67 | 94.73 |
| 224.0 | 6.90 | 95.63 |
| 219.2 | 7.67 | 97.43 |
| 210.0 | 9.01 | 97.83 |
| 202.5 | 10.31 | 97.24 |
| 196.5 | 11.59 | 98.39 |
| 175.2 | 17.56 | 98.99 |
| 149.3 | 30.04 | 99.64 |
| 137.2 | 38.47 | 99.76 |
| 136.8 | 38.95 | 98.78 |
| 131.8 | 43.58 | 99.76 |
| 121.5 | 56.33 | 99.84 |
| 112.8 | 70.68 | 99.93 |
| 111.3 | 73.86 | 99.94 |
| 106.3 | 84.42 | 99.96 |
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
- ↑ "Physical Properties of Glycerine and its solutions" (PDF). Retrieved 19 March 2021.
- ↑ Segur, J. B.; Oberstar, H. E. (1951). "Viscosity of Glycerol and Its Aqueous Solutions". Industrial & Engineering Chemistry. 43 (9): 2117–2120. doi:10.1021/ie50501a040.
- ↑ "Glycerin".
- ↑ Lange's Handbook of Chemistry, 17th Ed., 2017, McGraw-Hill Education, Table 2.54.
- ↑ "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 13 May 2007.
- ↑ Freezing Points of Glycerine-Water Solutions Dow Chemical
- ↑ Lane, Leonard B. (September 1925). "Freezing Points of Glycerol and Its Aqueous Solutions". Industrial & Engineering Chemistry. 17 (9): 924. doi:10.1021/ie50189a017.
- ↑ "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 7 June 2007.