 | |
| Names | |
|---|---|
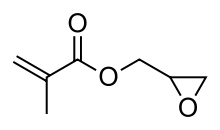
| IUPAC name
oxiran-2-ylmethyl 2-methylprop-2-enoate | |
| Other names
glycidyl methacrylate, 2,3-epoxypropyl methacrylate, 2-((Methacryloxy)methyl)oxirane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.130 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H10O3 | |
| Molar mass | 142.1546 g/mol |
| Appearance | colorless liquid |
| Density | 1.07 g/cm3 |
| Boiling point | 189.0 °C (372.2 °F; 462.1 K) |
| ca 50g/l | |
| Hazards | |
| Flash point | 76.0 °C (168.8 °F; 349.1 K) |
| 389.0 °C (732.2 °F; 662.1 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Glycidyl methacrylate (GMA) is an ester of methacrylic acid and glycidol. Containing both an epoxide and an acrylate groups, the molecule is bifunctional. It is a common monomer used in the production of epoxy resins. While typical home epoxies contain diglycidyl ether of bisphenol A (DGEBA), glycidyl methacrylate is instead used to provide epoxy functionalization to polyolefins and other acrylate resins. Glycidyl methacrylate is produced by several companies worldwide, including Dow Chemical.[2] It is used to prepare a range of composites.[3][4]
See also
References
- ↑ ChemExper.com
- ↑ Dow Chemical Marketing Page, retrieved November 2015
- ↑ Teng, Chih-Chun; Ma, Chen-Chi M.; Lu, Chu-Hua; Yang, Shin-Yi; Lee, Shie-Heng; Hsiao, Min-Chien; Yen, Ming-Yu; Chiou, Kuo-Chan; Lee, Tzong-Ming (2011). "Thermal conductivity and structure of non-covalent functionalized graphene/Epoxy composites". Carbon. 49 (15): 5107–5116. doi:10.1016/j.carbon.2011.06.095.
- ↑ Wang, Dong-An; Varghese, Shyni; Sharma, Blanka; Strehin, Iossif; Fermanian, Sara; Gorham, Justin; Fairbrother, D. Howard; Cascio, Brett; Elisseeff, Jennifer H. (2007). "Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration". Nature Materials. 6 (5): 385–392. doi:10.1038/nmat1890. PMC 8128046. PMID 17435762.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.