| H2BC21 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | H2BC21, GL105, H2B, H2B.1, H2BFQ, H2BGL105, H2BQ, histone cluster 2, H2be, histone cluster 2 H2B family member e, H2B clustered histone 21, HIST2H2BE, H2BE, H2B-GL105 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 601831 HomoloGene: 128426 GeneCards: H2BC21 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Histone H2B type 2-E is a protein that in humans is encoded by the HIST2H2BE gene.[3][4][5]
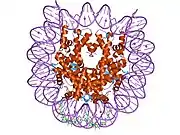
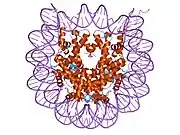
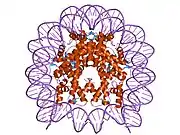
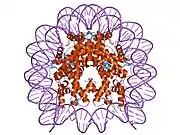
Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Two molecules of each of the four core histones (H2A, H2B, H3, and H4) form an octamer, around which approximately 146 bp of DNA is wrapped in repeating units, called nucleosomes. The linker histone, H1, interacts with linker DNA between nucleosomes and functions in the compaction of chromatin into higher order structures. This gene encodes a member of the histone H2B family, and generates two transcripts through the use of the conserved stem-loop termination motif, and the polyA addition motif.[5]
Cancer
HIST2H2BE gene has been detected progressively downregulated in Human papillomavirus-positive neoplastic keratinocytes derived from uterine cervical preneoplastic lesions at different levels of malignancy.[6] For this reason, this gene is likely to be associated with tumorigenesis and may be a potential prognostic marker for uterine cervical preneoplastic lesions progression.[6]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000184678 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Collart D, Romain PL, Huebner K, Pockwinse S, Pilapil S, Cannizzaro LA, Lian JB, Croce CM, Stein JL, Stein GS (Jan 1993). "A human histone H2B.1 variant gene, located on chromosome 1, utilizes alternative 3' end processing". J Cell Biochem. 50 (4): 374–85. doi:10.1002/jcb.240500406. PMID 1469070. S2CID 7732783.
- ↑ Marzluff WF, Gongidi P, Woods KR, Jin J, Maltais LJ (Oct 2002). "The human and mouse replication-dependent histone genes". Genomics. 80 (5): 487–98. doi:10.1016/S0888-7543(02)96850-3. PMID 12408966.
- 1 2 "Entrez Gene: HIST2H2BE histone cluster 2, H2be".
- 1 2 Rotondo JC, Bosi S, Bassi C, Ferracin M, Lanza G, Gafà R, Magri E, Selvatici R, Torresani S, Marci R, Garutti P, Negrini M, Tognon M, Martini F (April 2015). "Gene expression changes in progression of cervical neoplasia revealed by microarray analysis of cervical neoplastic keratinocytes". J Cell Physiol. 230 (4): 802–812. doi:10.1002/jcp.24808. hdl:11392/2066612. PMID 25205602. S2CID 24986454.
Further reading
- Collart D, Ramsey-Ewing A, Bortell R, et al. (1991). "Isolation and characterization of a cDNA from a human histone H2B gene which is reciprocally expressed in relation to replication-dependent H2B histone genes during HL60 cell differentiation". Biochemistry. 30 (6): 1610–7. doi:10.1021/bi00220a024. PMID 1993178.
- Jackson S, Brooks W, Jackson V (1994). "Dynamics of the interactions of histones H2A,H2B and H3,H4 with torsionally stressed DNA". Biochemistry. 33 (18): 5392–403. doi:10.1021/bi00184a006. PMID 8180162.
- Frohm M, Gunne H, Bergman AC, et al. (1996). "Biochemical and antibacterial analysis of human wound and blister fluid". Eur. J. Biochem. 237 (1): 86–92. doi:10.1111/j.1432-1033.1996.0086n.x. PMID 8620898.
- Rodriguez P, Munroe D, Prawitt D, et al. (1997). "Functional characterization of human nucleosome assembly protein-2 (NAP1L4) suggests a role as a histone chaperone". Genomics. 44 (3): 253–65. doi:10.1006/geno.1997.4868. PMID 9325046.
- El Kharroubi A, Piras G, Zensen R, Martin MA (1998). "Transcriptional Activation of the Integrated Chromatin-Associated Human Immunodeficiency Virus Type 1 Promoter". Mol. Cell. Biol. 18 (5): 2535–44. doi:10.1128/mcb.18.5.2535. PMC 110633. PMID 9566873.
- Zhang Y, Sun ZW, Iratni R, et al. (1998). "SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex". Mol. Cell. 1 (7): 1021–31. doi:10.1016/S1097-2765(00)80102-1. PMID 9651585.
- Lorain S, Quivy JP, Monier-Gavelle F, et al. (1998). "Core Histones and HIRIP3, a Novel Histone-Binding Protein, Directly Interact with WD Repeat Protein HIRA". Mol. Cell. Biol. 18 (9): 5546–56. doi:10.1128/MCB.18.9.5546. PMC 109139. PMID 9710638.
- Khan IU, Wallin R, Gupta RS, Kammer GM (1998). "Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane". Proc. Natl. Acad. Sci. U.S.A. 95 (18): 10425–30. Bibcode:1998PNAS...9510425K. doi:10.1073/pnas.95.18.10425. PMC 27910. PMID 9724719.
- Becker W, Weber Y, Wetzel K, et al. (1998). "Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases". J. Biol. Chem. 273 (40): 25893–902. doi:10.1074/jbc.273.40.25893. PMID 9748265.
- Allen MP, Zeng C, Schneider K, et al. (1999). "Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt". Mol. Endocrinol. 13 (2): 191–201. doi:10.1210/mend.13.2.0230. PMID 9973250.
- Piredda L, Farrace MG, Lo Bello M, et al. (1999). "Identification of 'tissue' transglutaminase binding proteins in neural cells committed to apoptosis". FASEB J. 13 (2): 355–64. doi:10.1096/fasebj.13.2.355. PMID 9973324. S2CID 16417170.
- Carrier F, Georgel PT, Pourquier P, et al. (1999). "Gadd45, a p53-Responsive Stress Protein, Modifies DNA Accessibility on Damaged Chromatin". Mol. Cell. Biol. 19 (3): 1673–85. doi:10.1128/MCB.19.3.1673. PMC 83961. PMID 10022855.
- Kawasaki H, Schiltz L, Chiu R, et al. (2000). "ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation". Nature. 405 (6783): 195–200. Bibcode:2000Natur.405..195K. doi:10.1038/35012097. PMID 10821277. S2CID 4330780.
- Deng L, de la Fuente C, Fu P, et al. (2001). "Acetylation of HIV-1 Tat by CBP/P300 increases transcription of integrated HIV-1 genome and enhances binding to core histones". Virology. 277 (2): 278–95. doi:10.1006/viro.2000.0593. PMID 11080476.
- Baake M, Doenecke D, Albig W (2001). "Characterisation of nuclear localisation signals of the four human core histones". J. Cell. Biochem. 81 (2): 333–46. doi:10.1002/1097-4644(20010501)81:2<333::AID-JCB1048>3.0.CO;2-D. PMID 11241673. S2CID 39592649.
- Chadwick BP, Willard HF (2001). "A Novel Chromatin Protein, Distantly Related to Histone H2a, Is Largely Excluded from the Inactive X Chromosome". J. Cell Biol. 152 (2): 375–84. doi:10.1083/jcb.152.2.375. PMC 2199617. PMID 11266453.
- Freire J, Covelo G, Sarandeses C, et al. (2001). "Identification of nuclear-import and cell-cycle regulatory proteins that bind to prothymosin alpha". Biochem. Cell Biol. 79 (2): 123–31. doi:10.1139/bcb-79-2-123. PMID 11310559.
- Nemergut ME, Mizzen CA, Stukenberg T, et al. (2001). "Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B". Science. 292 (5521): 1540–3. Bibcode:2001Sci...292.1540N. doi:10.1126/science.292.5521.1540. PMID 11375490.
- Deng L, Wang D, de la Fuente C, et al. (2001). "Enhancement of the p300 HAT activity by HIV-1 Tat on chromatin DNA". Virology. 289 (2): 312–26. doi:10.1006/viro.2001.1129. PMID 11689053.