 | |
| Names | |
|---|---|
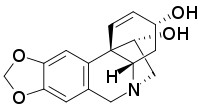
| Systematic IUPAC name
(3α,11R,13β)-1,2-Didehydrocrinan-3,11-diol | |
| Other names
Bulbispermine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H17NO4 | |
| Molar mass | 287.315 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hamayne is an alkaloid present in plants of the family Amaryllidaceae, including Iberian Narcissus species and two Nigerian Crinum species, reported to have acetylcholinesterase inhibitory activity.[1] The product has been made via total synthesis as well.[2]
References
- ↑ López, Susana; Bastida, Jaume; Viladomat, Francesc; Codina, Carles (2002). "Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts". Life Sciences. 71 (21): 2521–2529. doi:10.1016/S0024-3205(02)02034-9. PMID 12270757.
- ↑ Petit, Laurent; Banwell, Martin G.; Willis, Anthony C. (2011). "The Total Synthesis of the Crinine Alkaloid Hamayne via a Pd[0]-Catalyzed Intramolecular Alder-Ene Reaction". Organic Letters. 13 (21): 5800–5803. doi:10.1021/ol2023938. PMID 21970722.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.