| Lungless salamander Temporal range: | |
|---|---|
 | |
| Batrachoseps attenuatus | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Urodela |
| Suborder: | Salamandroidea |
| Family: | Plethodontidae Gray, 1850 |
| Subgroups | |
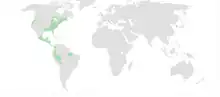 | |
| Native distribution of plethodontids (in green) | |
Plethodontidae, or lungless salamanders, are a family of salamanders.[1][2] Most species are native to the Western Hemisphere, from British Columbia to Brazil, although a few species are found in Sardinia, mainland Europe south of the Alps, and South Korea. In terms of number of species, they are by far the largest group of salamanders.[3]
Biology

Adult lungless salamanders have four limbs, with four toes on the fore limbs, and usually with five on the hind limbs. Within many species, mating and reproduction occur solely on land. Accordingly, many species also lack an aquatic larval stage, a phenomenon known as direct development in which the offspring hatch as fully-formed, miniature adults. Direct development is correlated with changes in the developmental characteristics of plethodontids compared to other families of salamanders including increases in egg size and duration of embryonic development. Additionally, the evolutionary loss of the aquatic larval stage is related to a diminishing dependence on aquatic habitats for reproduction. The lift of this constraint allowed widespread colonization and diversification within a broad number of terrestrial habitats which is a testament to the high success and proliferation of Plethodontidae.[4]
Many species have a projectile tongue and hyoid apparatus, which they can fire almost a body length at high speed to capture prey.
Measured in individual numbers, they are very successful animals where they occur. In some places, they make up the dominant biomass of vertebrates.[5] An estimated 1.88 billion individuals of the southern redback salamander inhabit just one district of Mark Twain National Forest alone, about 1,400 tons of biomass.[6] Due to their modest size and low metabolism, they are able to feed on prey such as springtails, which are usually too small for other terrestrial vertebrates. This gives them access to a whole ecological niche with minimal competition from other groups.
Courtship and mating
Plethodontids exhibit highly stereotyped and complex mating behaviors and courtship rituals that are not present in any other salamander family. Mating behavior tends to be uniform among all plethodontids and typically involves a tail-straddle walk in which the female orients her head at the base of the male's tail while also straddling the tail with her body. The male will twist his body around and deposit a sperm capsule, known as the spermatophore, on the substrate in front of the female's snout. As the male leads the female over the spermatophore with his tail, the female lowers her cloaca onto the spermatophore and lodges the sperm mass inside while leaving the base of the spermatophore on the ground.[7]
Within many species of plethodontidae, the courtship ritual is often accompanied by transfer of male pheromones during the tail-straddling walk. During the breeding period, males will grow enlarged anterior teeth used to scratch the female's skin on her head as a part of the courtship ritual. Subsequently, the male will rub pheromones onto the abraded spot which are secreted from a pad of tissue called the mental gland located underneath the male's chin.[7][8][9][10]
Courtship pheromones greatly increase male mating success for a variety of reasons. Overall, the pheromone secretions increase female receptivity to courtship and sperm transfer. This not only increases the likelihood of successful mating with a specific female, but also shortens the duration of courtship which is important because it minimizes the chance of the male being interrupted by other competing males.[11]
In scientific literature discussing the variations between the mental glands of plethodontid salamanders, it was discovered that male plethodontids had minor variations in height and diameter of the simple tubular glands, and major variation was found in the diameter of the secretory granules. This is attributed to the fact that males can mate throughout all months of the year, while females oviposit seasonally.
Respiration
A number of features distinguish the plethodontids from other salamanders. Most significantly, they lack lungs, conducting respiration through their skin, and the tissues lining their mouths.[3] Some species of cave salamanders are neotenic, and keep their larval gills even as adults. Gills are absent in all other adult plethodontids.[12]
Plethodontid salamanders are almost entirely reliant on cutaneous respiration.[13] Approximately 83%–93% of oxygen uptake is through this method.[14] Plethodontid salamander respiration rates are constrained by their SA:V, and higher SA:Vs are correlated to warmer, wetter climates.[15]
Plethodontids are constantly exposed to air or water, which allows for constant gas exchange that is not limited by ventilation.[13] Oxygen uptake is identical in water and air, assuming the partial pressure of oxygen is the same.[16] Oxygenated and non-oxygenated blood are mixed together in the venous system, which causes the partial pressure of oxygen within cardiac blood to typically be low.[13]
Plethodontids can tolerate hypoxia for prolonged periods by reducing their metabolic rate instead of by relying on anaerobic cutaneous respiration, as initially theorized.[16]
Plethodontids have been observed to develop rudimentary lungs as embryos.[17] The lung rudiment develops similarly to that of non-plethodontid salamanders for the first three weeks of development and then begins to regress through apoptosis.[17]
Chemoreception
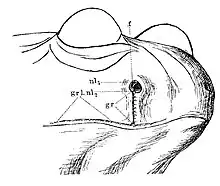
Another distinctive feature is the presence of a vertical slit between the nostril and upper lip, known as the "nasolabial groove". The groove is lined with glands, and enhances the salamander's chemoreception which is correlated with a higher degree of olfactory lobe and nasal mucous membrane development in plethodontids.[3][18] The presence of this specialized structure is likely related to the absence of lungs in these salamanders. Though some lunged salamanders do exhibit similar structures, they are reduced in size and are not arranged near the nostrils (i.e. nares) in the same fashion as plethodontids. Due to the fact that plethodontids cannot generate air pressure via expulsion of air from the lungs and through the nares, they are presented with the challenge of removing water and debris from the nasal passages which has the potential to significantly limit olfactory processes. As such, the nasolabial grooves are structured in a way that maximizes drainage from the nose. The groove is deeper and more narrow directly around the nares and the orifices of the glands are slightly elevated both of which aid in the gravitational flow of fluid from the nares and nasal depression. Additionally, the nasolabial glands around the margins of the nares secrete a fatty film which further encourages the removal of water from the nasal passages due to differences in polarity between water and the lipid secretions.[18]
Evolutionary history
Plethodontidae are estimated to have split from their sister group Amphiumidae around the K-Pg boundary, and to have diversified during the Paleogene.[19] The origin region of the family is North America, with oldest of the European members of the family known from the Middle Miocene of Slovakia.[20]
Subfamilies and genera
The family Plethodontidae consists of two extant subfamilies and about 506[2] to 510 species divided among these genera,[1] making up the majority of known salamander species:[2]
| Subfamily | Genus, scientific name, and author | Common name | Species |
|---|---|---|---|
| Hemidactyliinae Hallowell, 1856 | |||
| Aquiloeurycea Rovito, Parra-Olea, Recuero, and Wake, 2015 | 6 | ||
| Batrachoseps Bonaparte, 1839 | Slender salamanders | 21 | |
| Bolitoglossa Duméril, Bibron & Duméril, 1854 | Tropical climbing salamanders | 132 | |
| Bradytriton Wake & Elias, 1983 | Finca Chiblac salamander | 1 | |
| Chiropterotriton Taylor, 1944 | Splay-foot salamanders | 18 | |
| Cryptotriton García-París & Wake, 2000 | Hidden salamanders | 7 | |
| Dendrotriton Wake & Elias, 1983 | Bromeliad salamanders | 8 | |
| Eurycea Rafinesque, 1822 | North American brook salamanders | 32 | |
| Gyrinophilus Cope, 1869 | Spring salamanders | 4 | |
| Hemidactylium Tschudi, 1838 | Four-toed salamanders | 1 | |
| Isthmura Dubois and Raffaelli, 2012 | 7 | ||
| Ixalotriton Wake and Johnson, 1989 | Jumping salamanders | 2 | |
| Nototriton Wake & Elias, 1983 | Moss salamanders | 20 | |
| Nyctanolis Elias & Wake, 1983 | Long-limbed salamanders | 1 | |
| Oedipina Keferstein, 1868 | Worm salamanders | 38 | |
| Parvimolge Taylor, 1944 | Tropical dwarf salamanders | 1 | |
| Pseudoeurycea Taylor, 1944 | False brook salamanders | 39 | |
| Pseudotriton Tschudi, 1838 | Mud and red salamanders | 3 | |
| Stereochilus Cope, 1869 | Many-lined salamanders | 1 | |
| Thorius Cope, 1869 | Minute salamanders | 29 | |
| Urspelerpes Camp, Peterman, Milanovich, Lamb, Maerz, and Wake, 2009 | Patch-nosed salamanders | 1 | |
| Plethodontinae Gray, 1850 | |||
| Aneides Baird, 1851 | Climbing salamanders | 9 | |
| Desmognathus Baird, 1850 | Dusky salamanders | 30 | |
| Ensatina Gray, 1850 | Ensatinas | 1 | |
| Hydromantes Gistel, 1848 | Web-toed salamanders | 5 | |
| Karsenia Min, Yang, Bonett, Vieites, Brandon & Wake, 2005 | Korean crevice salamanders | 1 | |
| Phaeognathus Highton, 1961 | Red Hills salamanders | 1 | |
| Plethodon Tschudi, 1838 | Slimy and mountain salamanders | 57 | |
| Speleomantes Dubois, 1984 | European cave salamanders | 8 | |
Following a major revision in 2006, the genus Haideotriton was found to be a synonym of Eurycea, while the genus Lineatriton were made synonyms of Pseudoeurycea.[21]
A single hemidactyliine (Palaeoplethodon) is known from Miocene fossil remains preserved in Dominican amber, marking the only record of salamanders in the Caribbean.[22]
Conservation status
| Status | Number of Species |
|---|---|
| Least Concern | 94 |
| Near Threatened | 39 |
| Vulnerable | 60 |
| Endangered | 88 |
| Critically Endangered | 68 |
| Extinct | 1 |
| Data Deficient | 40 |
References
- 1 2 Frost, Darrel R. (2023). "Plethodontidae Gray, 1850". Amphibian Species of the World: An Online Reference. Version 6.1. American Museum of Natural History. doi:10.5531/db.vz.0001. Retrieved 23 April 2023.
- 1 2 3 "Plethodontidae". AmphibiaWeb. University of California, Berkeley. 2023. Retrieved 23 April 2023.
- 1 2 3 Lanza, B.; Vanni, S.; Nistri, A. (1998). Cogger, H.G.; Zweifel, R.G. (eds.). Encyclopedia of Reptiles and Amphibians. San Diego: Academic Press. pp. 74–75. ISBN 0-12-178560-2.
- ↑ Lewis, Zachary R.; Kerney, Ryan; Hanken, James (2011). "Lung development in lungless salamanders!". Developmental Biology. 356 (1): 250–251. doi:10.1016/j.ydbio.2011.05.560.
- ↑ Hairston, N.G., Sr. 1987. Community ecology and salamander guilds. Cambridge University Press. Cambridge.
- ↑ "Salamanders a more abundant food source in forest ecosystems than previously thought". ScienceDaily. 18 November 2014.
- 1 2 Sever, David M. (2003). Reproductive biology and phylogeny of Urodela. Enfield, NH: Science Publishers. pp. 383–424. ISBN 978-1-57808-645-0. OCLC 427511083.
- ↑ Sever, David M.; Dustin S. Siegel; Michael S. Taylor; Christopher K. Beachy1 (March 17, 2016). "Phylogeny of Mental Glands, Revisited". Copeia. 104 (1): 83–93. doi:10.1643/CH-14-210. PMC 6054469. PMID 30034038.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ David M. Sever (January 18, 2016). "Ultrastructure of the mental gland of the Red-Backed Salamander, Plethodon cinereus (Amphibia: Plethodontidae)". Acta Zoologica. 98 (2): 154–162. doi:10.1111/azo.12158.
- ↑ Ariana E. Rupp; David M. Sever (February 14, 2017). "Histology of mental and caudal courtship glands in three genera of plethodontid salamanders (Amphibia: Plethodontidae)" (PDF). Acta Zoologica. 98 (2): 154–162. doi:10.1111/azo.12188. Retrieved July 5, 2022.
- ↑ Houck, Lynne D.; Reagan, Nancy L. (1990). "Male courtship pheromones increase female receptivity in a plethodontid salamander". Animal Behaviour. 39 (4): 729–734. doi:10.1016/s0003-3472(05)80384-7. S2CID 53185123.
- ↑ Holman, J. Alan (2006). Fossil Salamanders of North America. Indiana University Press. ISBN 0253347327.
- 1 2 3 Gatz, Randall N.; Crawford, Eugene C.; Piiper, Johannes (1974-02-01). "Respiratory properties of the blood of a lungless and gill-less salamander, Desmognathus fuscus". Respiration Physiology. 20 (1): 33–41. doi:10.1016/0034-5687(74)90016-4. PMID 4821655.
- ↑ Whitford, Walter G. (1973). "The effects of temperature on respiration in the Amphibia". American Zoologist. 13 (2): 505–512. doi:10.1093/icb/13.2.505.
- ↑ Baken, Erica K.; Mellenthin, Lauren E.; Adams, Dean C. (2020). "Macroevolution of desiccation‐related morphology in plethodontid salamanders as inferred from a novel surface area to volume ratio estimation approach". Evolution. 74 (2): 476–486. doi:10.1111/evo.13898. PMID 31849047. S2CID 209407983.
- 1 2 Gatz, Randall N.; Crawford, Eugene C.; Piiper, Johannes (1974-02-01). "Metabolic and heart rate response of the plethodontid salamander Desmognathus fuscus to hypoxia". Respiration Physiology. 20 (1): 43–49. doi:10.1016/0034-5687(74)90017-6. PMID 4821656.
- 1 2 Lewis, Zachary R.; Kerney, Ryan; Hanken, James (2022-08-19). "Developmental basis of evolutionary lung loss in plethodontid salamanders". Science Advances. 8 (33): eabo6108. Bibcode:2022SciA....8O6108L. doi:10.1126/sciadv.abo6108. PMC 9385146. PMID 35977024.
- 1 2 Brown, Charles E.; Martof, Bernard S. (1966-10-01). "The Function of the Naso-Labial Groove of Plethodontid Salamanders". Physiological Zoology. 39 (4): 357–367. doi:10.1086/physzool.39.4.30152358. S2CID 87787255.
- ↑ Shen, Xing-Xing; Liang, Dan; Chen, Meng-Yun; Mao, Rong-Li; Wake, David B.; Zhang, Peng (January 2016). "Enlarged Multilocus Data set Provides Surprisingly Younger Time of Origin for the Plethodontidae, the Largest Family of Salamanders". Systematic Biology. 65 (1): 66–81. doi:10.1093/sysbio/syv061. PMID 26385618.
- ↑ Sanchíz, Borja; Venczel, Márton (2005). "A fossil plethodontid salamander from the Middle Miocene of Slovakia (Caudata, Plethodontidae)". Amphibia-Reptilia. 26 (3): 408–411. doi:10.1163/156853805774408586.
- ↑ Frost, D. R.; Grant, T.; Faivovich, J. N.; Bain, R. H.; Haas, A.; Haddad, C. F. B.; De Sá, R. O.; Channing, A.; Wilkinson, M.; Donnellan, S. C.; Raxworthy, C. J.; Campbell, J. A.; Blotto, B. L.; Moler, P.; Drewes, R. C.; Nussbaum, R. A.; Lynch, J. D.; Green, D. M. & Wheeler, W. C. (2006). "The amphibian tree of life". Bulletin of the American Museum of Natural History. 297: 1–291. doi:10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2. hdl:2246/5781.
- ↑ Poinar Jr., G.; Wake, David B. (2015). "Palaeoplethodon hispaniolae gen. n., sp. n. (Amphibia: Caudata), a fossil salamander from the Caribbean" (PDF). Palaeodiversity. 8: 21–29.
- ↑ IUCN RedList, (2020). Plethodontidae. Retrieved from https://www.iucnredlist.org/search?taxonomies=101246&searchType=species
