S-1_100.svg.png.webp)
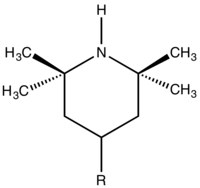
Hindered amine light stabilizers (HALS) are chemical compounds containing an amine functional group that are used as stabilizers in plastics and polymers.[1] These compounds are typically derivatives of tetramethylpiperidine and are primarily used to protect the polymers from the effects of photo-oxidation; as opposed to other forms of polymer degradation such as ozonolysis.[2][3] They are also increasingly being used as thermal stabilizers,[4] particularly for low and moderate level of heat, however during the high temperature processing of polymers (e.g. injection moulding) they remain less effective than traditional phenolic antioxidants.[5]
Mechanism of action
HALS do not absorb UV radiation, but act to inhibit degradation of the polymer by continuously and cyclically removing free radicals that are produced by photo-oxidation of the polymer. The overall process is sometimes referred to as the Denisov cycle, after Evguenii T. Denisov[6] and is exceedingly complex.[7] Broadly, HALS react with the initial polymer peroxy radical (ROO•) and alkyl polymer radicals (R•) formed by the reaction of the polymer and oxygen, preventing further radical oxidation. By these reactions HALS are oxidised to their corresponding aminoxyl radicals (R2NO• c.f. TEMPO), however they are able to return to their initial amine form via a series of additional radical reactions. HALS's high efficiency and longevity are due to this cyclic process wherein the HALS are regenerated rather than consumed during the stabilization process.
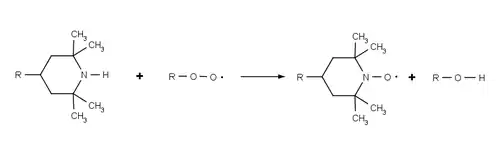
The structure of the HALS makes them resistant to side reactions. The use of a hindered amine possessing no alpha-hydrogens prevents the HALS being converted into a nitrone species and piperidines are resistant to intramolecular Cope reactions.[8] In commercial HALS the reactive piperidine group is usually bonded to bulky chemical scaffold, in order to reduce its volatility during the melt processing of plastic.
Application
Even though HALS are extremely effective in polyolefins, polyethylene and polyurethane, they are ineffective in polyvinyl chloride (PVC). It is thought that their ability to form nitroxyl radicals is disrupted due them being readily protonated by HCl released by dehydrohalogenation of PVC.
References
- ↑ Zweifel, Hans; Maier, Ralph D.; Schiller, Michael (2009). Plastics additives handbook (6th ed.). Munich: Hanser. ISBN 978-3-446-40801-2.
- ↑ Pieter Gijsman (2010). "Photostabilisation of Polymer Materials". In Norman S. Allen (ed.). Photochemistry and Photophysics of Polymer Materials Photochemistry. Hoboken: John Wiley & Sons. pp. 627–679. doi:10.1002/9780470594179.ch17. ISBN 978-0-470-59417-9..
- ↑ Klaus Köhler; Peter Simmendinger; Wolfgang Roelle; Wilfried Scholz; Andreas Valet; Mario Slongo (2010). "Paints and Coatings, 4. Pigments, Extenders, and Additives". Ullmann's Encyclopedia Of Industrial Chemistry. doi:10.1002/14356007.o18_o03. ISBN 978-3-527-30673-2.
- ↑ Gijsman, Pieter (November 2017). "A review on the mechanism of action and applicability of Hindered Amine Stabilizers". Polymer Degradation and Stability. 145: 2–10. doi:10.1016/j.polymdegradstab.2017.05.012.
- ↑ Gensler, R; Plummer, C.J.G; Kausch, H.-H; Kramer, E; Pauquet, J.-R; Zweifel, H (February 2000). "Thermo-oxidative degradation of isotactic polypropylene at high temperatures: phenolic antioxidants versus HAS". Polymer Degradation and Stability. 67 (2): 195–208. doi:10.1016/S0141-3910(99)00113-5.
- ↑ Denisov, E.T. (January 1991). "The role and reactions of nitroxyl radicals in hindered piperidine light stabilisation". Polymer Degradation and Stability. 34 (1–3): 325–332. doi:10.1016/0141-3910(91)90126-C.
- ↑ Hodgson, Jennifer L.; Coote, Michelle L. (25 May 2010). "Clarifying the Mechanism of the Denisov Cycle: How do Hindered Amine Light Stabilizers Protect Polymer Coatings from Photo-oxidative Degradation?". Macromolecules. 43 (10): 4573–4583. Bibcode:2010MaMol..43.4573H. doi:10.1021/ma100453d. hdl:1885/59767.
- ↑ March, Jerry; Smith, Michael B. (16 January 2007). March's advanced organic chemistry: reactions, mechanisms, and structure (6th. ed.). Wiley-Interscience. p. 1525. ISBN 978-0-471-72091-1.