| |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.998 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O2 | |
| Molar mass | 102.133 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 141 °C (286 °F; 414 K) |
| Hazards | |
| GHS labelling:[1] | |
 | |
| Warning | |
| H319 | |
| P264, P280, P305+P351+P338, P337+P313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
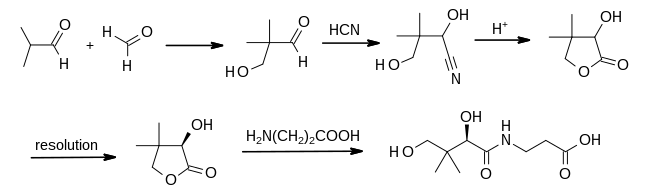
Hydroxypivaldehyde is the organic compound with the formula HOCH2(CH3)2CCHO. A colorless liquid, it is produced by condensation of formaldehyde and isobutyraldehyde:[2]
- CH2O + (CH3)2CHCHO → HOCH2(CH3)2CCHO
The compound is a rare example of a distillable aldol (3-hydroxyaldehyde). Upon standing, it dimerizes reversibly to the dioxane derivative.
Applications
Hydroxypivaldehyde is a precursor to vitamin B5 as is practiced commercially.[3]
Hydroxypivaldehyde is also a precursor to neopentyl glycol by hydrogenation:
- HOCH2(CH3)2CCHO + H2 → (CH3)2C(CH2OH)2
References
- ↑ "3-Hydroxy-2,2-dimethylpropanal". pubchem.ncbi.nlm.nih.gov. Retrieved 31 December 2021.
- ↑ Kohlpaintner, Christian; Schulte, Markus; Falbe, Jürgen; Lappe, Peter; Weber, Jürgen; Frey, Guido D. (2013). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub3. ISBN 978-3527306732.
- ↑ Eggersdorfer, Manfred; Laudert, Dietmar; Létinois, Ulla; McClymont, Tom; Medlock, Jonathan; Netscher, Thomas; Bonrath, Werner (2012). "One Hundred Years of Vitamins-A Success Story of the Natural Sciences". Angewandte Chemie International Edition. 51 (52): 12975. doi:10.1002/anie.201205886. PMID 23208776.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.