Hyperpolarization is the nuclear spin polarization of a material in a magnetic field far beyond thermal equilibrium conditions determined by the Boltzmann distribution.[1] It can be applied to gases such as 129Xe and 3He, and small molecules where the polarization levels can be enhanced by a factor of 104-105 above thermal equilibrium levels. Hyperpolarized noble gases are typically used in magnetic resonance imaging (MRI) of the lungs.[2] Hyperpolarized small molecules are typically used for in vivo metabolic imaging. For example, a hyperpolarized metabolite can be injected into animals or patients and the metabolic conversion can be tracked in real-time. Other applications include determining the function of the neutron spin-structures by scattering polarized electrons from a very polarized target (3He), surface interaction studies, and neutron polarizing experiments.[3]
Spin-exchange optical pumping
Introduction
Spin exchange optical pumping (SEOP)[3] is one of several hyperpolarization techniques discussed on this page. This technique specializes in creating hyperpolarized (HP) noble gases, such as 3He, 129Xe, and quadrupolar 131Xe, 83Kr, and 21Ne.[4] Noble gases are required because SEOP is performed in the gas phase, they are chemically inert, non-reactive, chemically stable with respect to alkali metals, and their T1 is long enough to build up polarization. Spin 1/2 noble gases meet all these requirements, and spin 3/2 noble gases do to an extent, although some spin 3/2 do not have a sufficient T1. Each of these noble gases has their own specific application, such as characterizing lung space and tissue via in vivo molecular imaging and functional imaging of lungs, to study changes in metabolism of healthy versus cancer cells,[4] or use as targets for nuclear physics experiments.[5] During this process, circularly polarized infrared laser light, tuned to the appropriate wavelength, is used to excite electrons in an alkali metal, such as caesium or rubidium inside a sealed glass vessel. Infrared light is necessary because it contains the wavelengths necessary to excite the alkali metal electrons, although the wavelength necessary to excite sodium electrons is below this region (Table 1).
| Alkali Metal | Wavelength (nm) |
|---|---|
| Sodium[6] | 590.0 |
| Rubidium[7] | 794.7 |
| Cesium[8] | 894.0 |
The angular momentum is transferred from the alkali metal electrons to the noble gas nuclei through collisions. Nitrogen is used as a quenching gas, which prevents the fluorescence of the polarized alkali metal, which would lead to de-polarization of the noble gas. If fluorescence was not quenched, the light emitted during relaxation would be randomly polarized, working against the circularly polarized laser light. While different sizes of glass vessels (also called cells), and therefore different pressures, are used depending on the application, one amagat of total pressure of noble gas and nitrogen is sufficient for SEOP and 0.1 amagat of nitrogen density is needed to quench fluorescence.[3] Great improvements in 129Xe hyperpolarization technology have achieved > 50% level at flow rates of 1–2 L/min, which enables human clinical applications.[9]
History
The discovery of SEOP took decades for all the pieces to fall into place to create a complete technique. First, in 1897, Zeeman's studies of sodium vapor led to the first result of optical pumping.[4][10] The next piece was found in 1950 when Kastler determined a method to electronically spin-polarize rubidium alkali metal vapor using an applied magnetic field and illuminating the vapor with resonant circularly polarized light.[4] Ten years later, Marie-Anne Bouchiat, T. M. Carver, and C. M. Varnum performed spin exchange, in which the electronic spin polarization was transferred to nuclear spins of a noble gas (3He and 129Xe) through gas-phased collisions.[4] Since then, this method has been greatly improved and expanded to use with other noble gases and alkali metals.
Theory
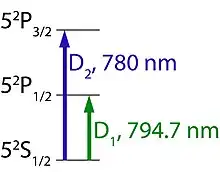
To explain the processes of excitation, optical pumping, and spin exchange easier, the most common alkali metal used for this process, rubidium, will be used as an example. Rubidium has an odd number of electrons, with only one in the outermost shell that can be excited under the right conditions. There are two transitions that can occur, one referred to as the D1 line where the transition occurs from the 52S1/2 state to the 52P3/2 state and another referred to the D2 line where the transition occurs from the 52S1/2 to the 52P1/2 state.[7][11] The D1 and D2 transitions can occur if the rubidium atoms are illuminated with light at a wavelength of 794.7 nm and 780 nm, respectively (Figure 1).[7] While it is possible to cause either excitation, laser technology is well-developed for causing the D1 transition to occur. Those lasers are said to be tuned to the D1 wavelength (794.7 nm) of rubidium.
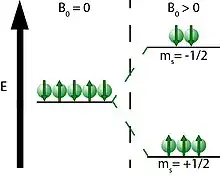
In order to increase the polarization level above thermal equilibrium, the populations of the spin states must be altered. In the absence of magnetic field, the two spin states of a spin I = ½ nuclei are in the same energy level, but in the presence of a magnetic field, the energy levels split into ms = ±1/2 energy levels (Figure 2).[12] Here, ms is the spin angular momentum with possible values of +1/2 (spin up) or -1/2 (spin down), often drawn as vectors pointing up or down, respectively. The difference in population between these two energy levels is what produces an NMR signal. For example, the two electrons in the spin down state cancel two of the electrons in the spin up state, leaving only one spin up nucleus to be detected with NMR. However, the populations of these states can be altered via hyperpolarization, allowing the spin up energy level to be more populated and therefore increase the NMR signal. This is done by first optically pumping alkali metal, then transferring the polarization to a noble gas nucleus to increase the population of the spin up state.

The absorption of laser light by the alkali metal is the first process in SEOP.[3] Left-circularly polarized light tuned to the D1 wavelength of the alkali metal excites the electrons from the spin down 2S1/2 (ms=-1/2) state into the spin up 2P1/2 (ms=+1/2) state, where collisional mixing then occurs as the noble gas atoms collide with the alkali metal atoms and the ms=-1/2 state is partially populated (Figure 3).[3] Circularly polarized light is necessary at low magnetic fields because it allows only one type of angular momentum to be absorbed, allowing the spins to be polarized.[3] Relaxation then occurs from the excited states (ms=±1/2) to the ground states (ms=±1/2) as the atoms collide with nitrogen, thus quenching any chance of fluorescence and causing the electrons to return to the two ground states in equal populations.[3] Once the spins are depolarized (return to the ms=-1/2 state), they are excited again by the continuous wave laser light and the process repeats itself. In this way, a larger population of electron spins in the ms=+1/2 state accumulates. The polarization of the rubidium, PRb, can be calculated by using the formula below:
Where n↑ and n↓ and are the number of atoms in the spin up (mS=+1/2) and spin down (mS=-1/2) 2S1/2 states.[13]

Next, the optically pumped alkali metal collides with the noble gas, allowing for spin exchange to occur where the alkali metal electron polarization is transferred to the noble gas nuclei (Figure 4). There are two mechanisms in which this can occur. The angular momentum can be transferred via binary collisions (Figure 4A, also called two-body collisions) or while the noble gas, N2 buffer gas, and vapor phase alkali metal are held in close proximity via van der Waals forces (Figure 4B, also called three body collisions).[3] In cases where van der Waals forces are very small compared to binary collisions (such is the case for 3He), the noble gas and alkali metal collide and polarization is transferred from the AM to the noble gas.[3] Binary collisions are also possible for 129Xe. At high pressures, van der Waals forces dominate, but at low pressures binary collisions dominate.[3]
Buildup of polarization
This cycle of excitation, polarization, depolarization, and re-polarization, etc. takes time before a net polarization is achieved. The buildup of nuclear polarization, PN(t), is given by:
Where ⟨PA⟩ is the alkali metal polarization, γSE is the spin exchange rate, and Γ is the longitudinal relaxation rate of the noble gas.[14] Relaxation of the nuclear polarization can occur via several mechanisms and is written as a sum of these contributions:
Where Γt, Γp, Γg, and Γw represent the relaxation from the transient Xe2 dimer, the persistent Xe2 dimer, diffusion through gradients in the applied magnetic field, and wall relaxation, respectively.[14] In most cases, the largest contributors to the total relaxation are persistent dimers and wall relaxations.[14] A Xe2 dimer can occur when two Xe atoms collide and are held together via van der Waals forces, and it can be broken when a third atom collides with it.[15] It is similar to Xe-Rb during spin exchange (spin transfer) where they are held in close proximity to each other via van der Waals forces.[15] Wall relaxation is when the hyperpolarized Xe collides with the walls of the cell and is de-polarized due to paramagnetic impurities in the glass.
The buildup time constant, ΓB, can be measured by collecting NMR spectra at time intervals falling within the time it takes to reach steady-state polarization (i.e. the maximum polarization that can be achieved, seen by the maximum signal output). The signal integrals are then plotted over time and can be fit to obtain the buildup time constant. Collecting a buildup curve at several different temperatures and plotting the values as a function of alkali metal vapor density (since vapor density increases with an increase in cell temperature) can be used to determine the spin destruction rate and the per-atom spin exchange rate using:
Where γ' is the per-atom spin exchange rate, [AM] is the alkali metal vapor density, and ΓSD is the spin destruction rate.[16] This plot should be linear, where γ' is the slope and ΓSD is the y-intercept.
Relaxation: T1
Spin exchange optical pumping can continue indefinitely with continuous illumination, but there are several factors that cause relaxation of polarization and thus a return to the thermal equilibrium populations when illumination is stopped. In order to use hyperpolarized noble gases in applications such as lung imaging, the gas must be transferred from the experimental setup to a patient. As soon as the gas is no longer actively being optically pumped, the degree of hyperpolarization begins to decrease until thermal equilibrium is reached. However, the hyperpolarization must last long enough to transfer the gas to the patient and obtain an image. The longitudinal spin relaxation time, denoted as T1, can be measured easily by collecting NMR spectra as the polarization decreases over time once illumination is stopped. This relaxation rate is governed by several depolarization mechanisms and is written as:
Where the three contributing terms are for collisional relaxation (CR), magnetic field inhomogeneity (MFI) relaxation, and relaxation caused by the presence of paramagnetic oxygen (O2).[17] The T1 duration could be anywhere from minutes to several hours, depending on how much care is put into lessening the effects of CR, MFI, and O2. The last term has been quantified to be 0.360 s−1 amagat−1,[18] but the first and second terms are hard to quantify since the degree of their contribution to the overall T1 is dependent on how well the experimental setup and cell are optimized and prepared.[18]
Experimental setup in SEOP

In order to perform SEOP, it is first necessary to prepare the optical cell. Optical cells (Figure 5) are designed for the particular system in mind and glass blown using a transparent material, typically pyrex glass (borosilicate). This cell must then be cleaned to eliminate all contaminants, particularly paramagnetic materials which decrease polarization and the T1. The inner surface of the cell is then coated to (a) serve as a protective layer for the glass in order to lessen the chance of corrosion by the alkali metal, and (b) minimize depolarization caused by the collisions of polarized gas molecules with the walls of the cell.[19] Decreasing wall relaxation leads to longer and higher polarization of the noble gas.[19]

While several coatings have been tested over the years, SurfaSil (Figure 6, now referred to as hydrocarbon soluble siliconizing fluid) is the most common coating used in a ratio of 1:10 SurfaSil: hexane because it provides long T1 values.[19] The thickness of the SurfaSil layer is about 0.3-0.4 μm.[19] Once evenly coated and dried, the cell is then placed in an inert environment and a droplet of alkali metal (≈200 mg) is placed in the cell, which is then dispersed to create an even coating on the walls of the cells. One method for transferring the alkali metal into the cell is by distillation.[20] In the distillation method, the cell is connected to a glass manifold equipped to hold both pressurized gas and vacuum, where an ampoule of alkali metal is connected.[21] The manifold and cell are vacuumed, then the ampoule seal is broken and the alkali metal is moved into the cell using the flame of a gas torch.[21] The cell is then filled with the desired gas mixture of nitrogen and noble gas.[5] Care must be taken not to poison the cell at any stage of cell preparation (expose the cell to atmospheric air).
Several cell sizes and designs have been used over the years. The application desired is what governs the design of the optical pumping cell and is dependent on laser diameter, optimization needs, and clinical use considerations. The specific alkali metal(s) and gases are also chosen based on the desired applications.

Once the cell is complete, a surface coil (or coils, depending on the desired coil type) is taped to the outside of the cell, which a) allows RF pulses to be produced in order to tip the polarized spins into the detection field (x,y plane) and b) detects the signal produced by the polarized nuclear spins. The cell is placed in an oven which allows for the cell and its contents to be heated so the alkali metal enters the vapor phase, and the cell is centered in a coil system which generates an applied magnetic field (along the z-axis). A laser, tuned to the D1 line (electric-dipole transition[14]) of the alkali metal and with a beam diameter matching the diameter of the optical cell, is then aligned with the optical flats of the cell in such a way where the entirety of the cell is illuminated by laser light to provide the largest polarization possible (Figure 7). The laser can be anywhere between tens of watts to hundreds of watts,[3] where higher the power yields larger polarization but is more costly. In order to further increase polarization, a retro-reflective mirror is placed behind the cell in order to pass the laser light through the cell twice. Additionally, an IR iris is placed behind the mirror, providing information of laser light absorption by the alkali metal atoms. When the laser is illuminating the cell, but the cell is at room temperature, the IR iris is used to measure the percent transmittance of laser light through the cell. As the cell is heated, the rubidium enters the vapor phase and starts to absorb laser light, causing the percent transmittance to decrease. The difference in the IR spectrum between a room temperature spectrum and a spectrum taken while the cell is heated can be used to calculate an estimated rubidium polarization value, PRb.
As SEOP continues to develop and improve, there are several types of NMR coils, ovens, magnetic field generating coils, and lasers that have been and are being used to generate hyperpolarized gases. Generally, the NMR coils are hand made for the specific purpose, either by turning copper wire by hand in the desired shape,[22] or by 3D printing the coil.[23] Commonly, the oven is a forced-air oven, with two faces made of glass for the laser light to pass through the cell, a removable lid, and a hole through which a hot air line is connected, which allows the cell to be heated via conduction.[24] The magnetic field generating coils can be a pair of Helmholtz coils, used to generate the desired magnetic field strength,[24] whose desired field is governed by:
Where ω is the Larmour frequency, or desired detection frequency, γ is the gyromagnetic ratio of the nuclei of interest, and B0 is the magnetic field required to detect the nuclei at the desired frequency.[25] A set of four electromagnetic coils can also be used (i.e. from Acutran)[22] and other coil designs are being tested.
In the past, laser technology was a limiting factor for SEOP, where only a couple alkali metals could be used due to the lack of, for example, cesium lasers. However, there have been several new developments, including better cesium lasers, higher power, narrower spectral width, etc. which are allowing the reaches of SEOP to increase. Nevertheless, there are several key features required. Ideally, the laser should be continuous wave to ensure the alkali metal and noble gas remains polarized at all times. In order to induce this polarization, the laser light must be circularly polarized in the direction which allows the electrons to become spin polarized. This is done by passing the laser light through a polarizing beam splitter to separate the s and p components, then through a quarter wave plate, which converts the linearly polarized light into circularly polarized light.[17]
Noble gases and alkali metals
SEOP has successfully been used and is fairly well developed for 3He, 129Xe, and 83Kr for biomedical applications.[4] Additionally, several improvements are under way to get enhanced and interpretable imaging of cancer cells in biomedical science.[26] Studies involving hyperpolarization of 131Xe are underway, piquing the interest of physicists. There are also improvements being made to allow not only rubidium to be utilized in the spin transfer, but also cesium. In principle, any alkali metal can be used for SEOP, but rubidium is usually preferred due to its high vapor pressure, allowing experiments to be carried out at relatively low temperatures (80 °C-130 °C), decreasing the chance of damaging the glass cell.[3] Additionally, laser technology for the alkali metal of choice has to exist and be developed enough get substantial polarization. Previously, the lasers available to excite the D1 cesium transition were not well-developed, but they are now becoming more powerful and less expensive. Preliminary studies even show that cesium may provide better results than rubidium, even though rubidium has been the go-to alkali metal of choice for SEOP.
The hyperpolarization method called spin-exchange optical pumping (SEOP) is being used to hyperpolarize noble gases such as Xenon-129 and Helium-3. When an inhaled hyperpolarized gas like 3He or 129Xe is imaged, there is a higher magnetization density of NMR-active molecules in the lung compared to traditional 1H imaging, which improves the MRI images that can be obtained. Unlike proton MRI which reports on anatomical features of lung tissues, XenonMRI reports lung function including gas ventilation, diffusion, and perfusion.[27]
Rationale
Our target is to identify the infection or disease (cancer, for example) anywhere in our body like cerebral, brain, blood, and fluid, and tissues. This infectious cell is called collectively biomarker.[28] According to the World Health Organization (WHO) and collaborating with United Nations and International Labor organization have convincingly defined the Biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. Biomarker has to be quantifiable up-to certain level in biological process in well-being.[28]
One specific example of biomarker is blood cholesterol that is commonly acquainted with us reliable for coronary heart disease; another biomarker is PSA (Prostate-Specific Antigen) and has been contributing to prostate cancer.[28] There are a lot of biomarkers are considering as being cancer: Hepatitis C virus ribonucleic acid (HCV-RNA), International Normalized Ratio (INR), Prothrombin Time (PT), Monoclonal Protein (M protein), Cancer Antigen-125 (CA-125), Human Immunodeficiency Virus -Ribonucleic Acid (HIV RNA), B-type Natriuretic Peptide (BNP).27and Lymphoma cell (Ramos cell lines and Jurkat cell lines) a form of cancer.[29]
Other common biomarkers are breast cancer, Ovarian cancer, Colorectal cancer, Lung cancer and brain tumor.[30]
This disease-causing verdict agent is the biomarker is existing extremely trace amount especially initial state of the disease. Therefore, identifying or getting images of biomarker is tricky and, in few circumstances, uncertain by NMR tech. Hence, we must use the contrasting agent to enhance the images at least to visualize level to Physicians. As molecules of biomarker is less abundant in vivo system. The NMR or MRI experiment provides a very small signal even in some cases, the analyzer can miss the signal peak in data due to the lack in abundance of biomarkers. Therefore, to make sure, to reach the true conclusion about the existence of trouble-causing biomarkers, we need to enhance the probe (contrasting mechanisms) to get the clear peak at the most visible level of peak height as well as the position of the peak in data. If it is possible to gather the acceptable and clearly interpretable data from NMR or MRI experiment by using the contrasting agent, then experts can take a right initial step to recover the patients who already have been suffering from cancer.[28] Among the various technique to get the enhanced data in MRI experiment, SEOP is one of them.
Researchers in SEOP are interested to use the 129Xe. Because 129Xe has a number of favorable facts in NMR Tech. for working as a contrasting agent even over the other novel gases:
- Inert xenon does not show chemical reaction like other metals and non-metals because Xenon's electronic configuration is fully occupied as well as it is not radioactive also.
- To get the solid, liquid state from naturally occurring gaseous state is easy going (figure-8). The solid and liquid state of 129Xe are existing experimentally doable temperature and pressure ranges.
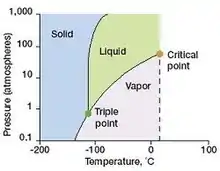 Figure 8. Diagram above shows the highest temperature and pressure at which xenon gas can exist in liquid and gaseous states simultaneously.30
Figure 8. Diagram above shows the highest temperature and pressure at which xenon gas can exist in liquid and gaseous states simultaneously.30 - Xenon possesses highly polarizable electron cloud surrounding the nucleus. Therefore, easily prone to be soluble with lipid or organic compounds especially in vivo environment in biological respect. (table-2)
- Xenon does not alter the structurally or chemically (similarly other noble gases) when interacting with other molecules.
- According to the scientist Ostwald, solubility is defined as the partition coefficient of the gas absorbed to the volume of the absorbing liquid. The solubility of Xenon, SXe(g) = V absorbed amount of Xe(g) /V absorbing liquid at standard temperature and pressure (STP).
Solubility of Xenon in water medium 11% means at 25 °C 11 mL Xenon gas could be absorbed by 100 mL of water.
| Name of Solvent Compound | Temperature (°C) | Ostwald Solubility in (v/v)% |
|---|---|---|
| Water | 25 | 0.11 |
| Hexane | 25 | 4.8 |
| Benzene | 25 | 3.1 |
| Fluorobenzene | 25 | 3.3 |
| Carbon Disulfide | 25 | 4.2 |
| Water | 37 | 0.08 |
| Saline | 37 | 0.09 |
| Plasma | 37 | 0.10 |
| Erythorcytes (98%) | 37 | 0.20 |
| Human albumin (100% extrapolated) | 37 | 0.15 |
| Blood | 37 | 0.14 |
| Oil | 37 | 1.90 |
| Fat tissue | 37 | 1.30 |
| DMSO | 37 | 0.66 |
| Intralipid (20%) | 37 | 0.40 |
| PFOB (perflubron) | 37 | 1.20 |
| PFOB (90% w/v, estimated) | 37 | 0.62 |
- Xenon atomic size is large and outer shell electrons are far from the nuclei, outermost electron is highly prone to be polarized specially lipid environment. Table 2 shows Xenon solubility in water medium at 37 °C is 8% but fat tissue in vivo environment the solubility value is 130%. Solubility leads the Xenon using in biological system as a contrasting agent.
- Solvent effect of xenon is very large for of 129Xenon by the fact of solubility (table 2). Chemical shift value range for Xenon is more than 7500 ppm. However solvent effect is limited range for 1H & 13C (MRI active nuclei) because of low chemical shift value range for 1H is 20 ppm and for 13C is 300 ppm. Therefore, using the 129Xe is preferred.
Figure-9 below, In NMR experimental data, there are different chemical shift values for different tissues in in vivo environment. All peaks are positioned through such a big range of chemical shift values for 129Xe is viable. Because 129Xe has long range up-to 1700ppm chemical shift value range in NMR data. Other important spectral information includes:

Figure 9. NMR data for Xe-129 biosensor in in vivo biological system.
- Naturally 129Xe NMR peak has been counted as reference at 0.0ppm.
- When 129Xe incorporated and bind with the Cryptophane-A molecule, then the chemical shift value in NMR acquisition shifted to around 70ppm.
- If hyperpolarized 129Xe gas is dissolved into the brain, then five NMR spectral peaks can be observed.[31]
- Among them top sharp peak at 194.7ppm. In addition, at 189 ppm peak come out from the non-brain tissues.
- Another two peaks are still unknown at 191.6 ppm and 197.8 ppm. At 209.5 ppm smaller but broad peak has been found in NMR data when 129Xe was dissolved in the blood stream.
- Hyperpolarized 129Xe is very sensitive detector of biomarker (form of cancer in living system).
- The nuclear spin polarization of 129Xe or in generally for noble gases we can increase up to fivefold via SEOP technique.[3]
- Using SEOP hyperpolarization technique, we can get images of uptake of xenon in the human brain tissue.[32]

(Figure-10) 129Xe(g) shows satisfactory enhancement in polarization during SEOP compared to the thermal enhancement in polarization. This is demonstrated by the experimental data values when NMR spectra are acquired at different magnetic field strengths.[22] A couple of important points from experimental data are:
- The 129Xe polarization has increased about 144,000-fold in SEOP tech. over thermally enhanced for 1H polarization in NMR experiment. Both experiments that showed this have been done in identical conditions and using same radio frequency during NMR experiment.[22]
- A similar value of 140,000-fold signal enhancement for 129Xe hyperpolarization in SEOP compare to the reference thermally enhanced 13C NMR signal is also seen in experimental NMR data. Both data have been collected in identical Larmor frequency and other experimental conditions and same radio frequency during NMR data collection.[22]
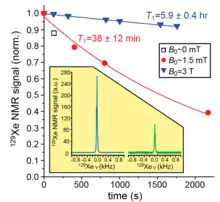
(Figure 11) Longitudinal spin relaxation time (T1) is very sensitive with an increase of magnetic field and hence enhance the NMR signals is noticeable in SEOP in case of 129Xe.[22] As T1 is higher for blue marking conditioning NMR experiment shows more enhanced peak compare to other.[22] For hyperpolarized 129Xe in tedlar bags, the T1 is 38±12 minutes when data collected in presence of 1.5 mT magnetic field. However, satisfactory increment in T1delay time (354±24 minutes) when data was collected in presence of 3000 mT magnetic field.[22]
Use of Rb vs. Cs in SEOP NMR experiments
In general, we can use the either 87Rb or 133Cs alkali metal atoms with inert nitrogen gas. However, we are using 133Cs atoms with nitrogen to make the spin exchange with 129Xe for number of advantages:
- 133Cs has natural perfect abundance while rubidium has two (85Rb and 87Rb) isotopes. Abstraction of one isotope separately from these two (85Rb and 87Rb) is difficult compare to collect the 133Cs isotope. Abstraction of 133Cs is convenient.
- Optical pumping cell normally is operated at lower temperature to avoid chemically breakdown issue. SEOP is using 133Cs at low temperature and hence it has fewer chemical corrosion with SEOP cell wall glass.
- 133Cs-129Xe couple have spin exchange rate about 10% that is more compare to the 87Rb-129Xe couple have.
Although 129Xe has a bunch of preferable characteristic applications in NMR technique, 83Kr can also be used since it has a lot of advantages in NMR techniques in different ways than 129Xe.
- 83Kr stable isotope has spin I=9/2 and has larger Van der Waals size 2.02A0. It has quadrupolar effect can be diffuse to nearby environment shortly & distinctively (polar to nonpolar media in vivo system).[33]
- Chemical composition of materials can influence the longitudinal relaxation of hyperpolarized 83Kr.[33]
- The relaxation can distinguish among the hydrophobic and hydrophilic substrate. Although 3He and 129Xe have spin half but they are not quadrupolar active.[33]
- However, the 21Ne (I=3/2), 83Kr(I=9/2) and 131Xe (I=3/2) have Quadrupolar moment.34 Quadrupolar interactions make these isotopes having spin relaxation.[33]
- Due to this spin relaxation and evolution, these isotopes can be used as contrasting agents to say about the probe can determine the structural feature and chemical compositions of the surfaces for a permeable media.[33]
- SEOP can calculate the relaxation of spin T1 by using the equation nonlinear least-squares fitting for 83Kr signal as a function of time as well as experimental number of media flip angle (≈12°) for NMR experimenting radio frequency pulses.[33]
- Hyperpolarized 83Kr is being separated from 87Rb gases after spin exchanging in the optical pumping process and then used in variety of in vivo system to get MRI signal. This is the first isotope showed lots of applicability for MRI technique even though has the spin is 9½.[33]
- During experiment of canine lung tissue, the using magnet was 9.4 T, media was porous and similar porosity to alveolar dimensions which is disseminated at atmospheric pressure. Spin lattice relaxation was reasonably long enough so it is applicable in vivo system although the oxygen level could be 20%.[33]
- As 83Kr contrasting agent is promising to develop pristine in vivo MRI methodology to identify the lung diseases epically those effect have been caused in parenchyma surface due to the surfactant concentration.[33]
- Boyed the boundary this particular contrasting agent can work to figure out the size of pour of porous media in materials science.[33]
- In addition, this technique can take us about to prepare the surface coating, spatial fluctuations of surfaces. Eventually, never ending the good sign of this contrasting agent like natural abundance (11.5% of 83Kr) makes it easy to get with reasonable price $5/L.[33]
Imaging applications of SEOP
Steps are also being taken in academia and industry to use this hyperpolarized gas for lung imaging. Once the gas (129Xe) is hyperpolarized through the SEOP process and the alkali metal is removed, a patient (either healthy or suffering from a lung disease), can breathe in the gas and an MRI can be taken.[34] This results in an image of the spaces in the lungs filled with the gas. While the process to get to the point of imaging the patient may require knowledge from scientists very familiar with this technique and the equipment, steps are being taken to eliminate the need for this knowledge so that a hospital technician would be able to produce the hyperpolarized gas using a polarizer.[22][23]
Hyperpolarization machines are currently being used to develop hyperpolarized xenon gas that is used as a visualization agent for the lungs. Xenon-129 is a safe inert noble gas that can be used to quantify lung function. With a single 10-second breath hold, hyperpolarized Xenon-129 is used with MRI to enable 3-dimensional lung imaging.[35] Xenon MRI is being used to monitor patients with pulmonary-vascular, obstructive, or fibrotic lung disease.[36]
Temperature-ramped 129Xe SEOP in an automated high-output batch model hyperpolarized 129Xe can utilize three prime temperature range to put certain conditions: First, 129Xe hyperpolarization rate is superlative high at hot condition. Second, in warm condition the hyperpolarization of 129Xe is unity. Third, at cold condition, the level of hyperpolarization of 129Xe gas at least can get the (at human body's temperature) imaging although during the transferring into the Tedlar bag having poor percentage of 87Rb (less than 5 ng/L dose).[37]
Multiparameter analysis of 87Rb/129Xe SEOP at high xenon pressure and photon flux could be used as 3D-printing and stopped flow contrasting agent in clinical scale.[38] In situ technique, the NMR machine was run for tracking the dynamics of 129Xe polarization as a function of SEOP-cell conditioning with different operating parameters such as data collecting temperature, photon flux, and 129Xe partial pressure to enhance the 129Xe polarization (PXe).[38]
| PXe | 95±9% | 73±4% | 60±2% | 41±1% | 31±1% |
| Partial pressure of Xe (torr) | 275 | 515 | 1000 | 1500 | 2000 |
All of those polarization values of 129Xe has been approved by pushing the hyperpolarized 129Xe gas and all MRI experiment also done at lower magnetic field 47.5 mT.[38] Finally demonstrations indicated that such a high pressure region, polarization of 129Xe gases could be increment even more that the limit that already has been shown. Better SEOP thermal management and optimizing the polarizing kinetics has been further improved with good efficacy.[38]
SEOP on solids
Not only can SEOP be used to hyperpolarize noble gases, but a more recent development is SEOP on solids. It was first performed in 2007[21] and was used to polarize nuclei in a solid, allowing for nuclei that cannot be polarized by other methods to become hyperpolarized.[21] For example, nuclear polarization of 133Cs in the form of a solid film of CsH can be increased above the Boltzmann limit.[21] This is done by first optically pumping cesium vapor, then transferring the spin polarization to CsH salt, yielding an enhancement of 4.0.[21]
The cells are made as previously described using distillation, then filled with hydrogen gas and heated to allow for the Cs metal to react with the gaseous hydrogen to form the CsH salt.[21] Unreacted hydrogen was removed, and the process was repeated several times to increase the thickness of the CsH film, then pressurized with nitrogen gas.[21] Usually, SEOP experiments are done with the cell centered in Helmholtz or electromagnetic coils, as previously described, but these experiments were done in a superconducting 9.4 T magnet by shining the laser through the magnet and electrically heating the cell.[21] In the future, it may be possible to use this technique to transfer polarization to 6Li or 7Li, leading to even more applications since the T1 is expected to be longer.[21] Since the discovery of this technique that allows solids to be characterized, it has been improved in such a way where polarized light is not necessary to polarize the solid; instead, unpolarized light in a magnetic field can be used.[39] In this method, glass wool is coated with CsH salt, increasing the surface area of the CsH and therefore increasing the chances of spin transfer, yielding 80-fold enhancements at low field (0.56 T).[39] Like in hyperpolarizing CsH film, the cesium metal in this glass wool method was allowed to react with hydrogen gas, but in this case the CsH formed on the glass fibers instead of the glass cell.[39]
Metastability exchange optical pumping
3He can also be hyperpolarized using metastability exchange optical pumping (MEOP). This process is able to polarize 3He nuclei in the ground state with optically pumped 3He nuclei in the metastable state. MEOP only involves 3He nuclei at room temperature and at low pressure (≈a few mbars). The process of MEOP is very efficient (high polarization rate), however, compression of the gas up to atmospheric pressure is needed.
Dynamic nuclear polarization
Compounds containing NMR-sensitive nuclei, such as 1H, 13C or 15N, can be hyperpolarized using Dynamic nuclear polarization (DNP). DNP is typically performed at low temperature (≈1 K) and high magnetic field (≈3 T). The compound is subsequently thawed and dissolved to yield a room temperature solution containing hyperpolarized nuclei.[40] This liquid can be used in in vivo metabolic imaging[41] for oncology[42] and other applications. The 13C polarization levels in solid compounds can reach up to ≈64% and the losses during dissolution and transfer of the sample for NMR measurements can be minimized to a few percent.[43] Compounds containing NMR-active nuclei can also be hyperpolarized using chemical reactions with para-hydrogen, see Para-Hydrogen Induced Polarization (PHIP).
Parahydrogen induced polarization
Molecular hydrogen, H2, contains two different spin isomers, para-hydrogen and ortho-hydrogen, with a ratio of 25:75 at room temperature. Creating para-hydrogen induced polarization (PHIP)[44] means that this ratio is increased, in other words that para-hydrogen is enriched. This can be accomplished by cooling hydrogen gas and then inducing ortho-to-para conversion via an iron-oxide or charcoal catalyst. When performing this procedure at ≈70 K (i.e. with liquid nitrogen), para-hydrogen is enriched from 25% to ca. 50%. When cooling to below 20 K and then inducing the ortho-to-para conversion, close to 100% parahydrogen can be obtained.
For practical applications, the PHIP is most commonly transferred to organic molecules by reacting the hyperpolarized hydrogen with precursor molecules in the presence of a transition metal catalyst. Proton NMR signals with ca. 10,000-fold increased intensity[45] can be obtained compared to NMR signals of the same organic molecule without PHIP and thus only "thermal" polarization at room temperature.
Signal amplification by reversible exchange (SABRE)
Signal amplification by reversible exchange (SABRE) is a technique to hyperpolarize samples without chemically modifying them. Compared to orthohydrogen or organic molecules, a much greater fraction of the hydrogen nuclei in parahydrogen align with an applied magnetic field. In SABRE, a metal center reversibly binds to both the test molecule and a parahydrogen molecule facilitating the target molecule to pick up the polarization of the parahydrogen.[46] This technique can be improved and utilized for a wide range of organic molecules by using an intermediate "relay" molecule like ammonia. The ammonia efficiently binds to the metal center and picks up the polarization from the parahydrogen. The ammonia then transfers it other molecules that don't bind as well to the metal catalyst.[47] This enhanced NMR signal allows the rapid analysis of very small amounts of material.
See also
References
- ↑ Leawoods, Jason C.; Yablonskiy, Dmitriy A.; Saam, Brian; Gierada, David S.; Conradi, Mark S. (2001). "Hyperpolarized 3He Gas Production and MR Imaging of the Lung". Concepts in Magnetic Resonance. 13 (5): 277–293. CiteSeerX 10.1.1.492.8128. doi:10.1002/cmr.1014.
- ↑ Altes, Talissa; Salerno, Michael (2004). "Hyperpolarized Gas Imaging of the Lung". J Thorac Imaging. 19 (4): 250–258. doi:10.1097/01.rti.0000142837.52729.38. PMID 15502612.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Walker, Thad G.; Happer, William (1997-04-01). "Spin-exchange optical pumping of noble-gas nuclei". Reviews of Modern Physics. 69 (2): 629–642. Bibcode:1997RvMP...69..629W. doi:10.1103/revmodphys.69.629. ISSN 0034-6861.
- 1 2 3 4 5 6 Nikolaou, Panayiotis; Goodson, Boyd M.; Chekmenev, Eduard Y. (2015-02-06). "Inside Cover: NMR Hyperpolarization Techniques for Biomedicine (Chem. Eur. J. 8/2015)". Chemistry - A European Journal. 21 (8): 3134. doi:10.1002/chem.201590031. ISSN 0947-6539.
- 1 2 Chupp, T. E.; Coulter, K. P. (1985-09-02). "Polarization ofNe21by Spin Exchange with Optically Pumped Rb Vapor". Physical Review Letters. 55 (10): 1074–1077. doi:10.1103/physrevlett.55.1074. ISSN 0031-9007. PMID 10031721.
- ↑ Steck, D. A., Sodium D Line Data. Oregon Center for Optics and Department of Physics, University of Oregon, 2000.
- 1 2 3 Steck, D. A., Rubidium 85 D Line Data. Oregon Center for Optics and Department of Physics, University of Oregon, 2013.
- ↑ Steck, D. A., Cesium D Line Data. Oregon Center for Optics and Department of Physics, University of Oregon, 2010.
- ↑ F. William Hersman; et al. (2008). "Large Production System for Hyperpolarized 129Xe for Human Lung Imaging Studies". Acad. Radiol. 15 (6): 683–692. doi:10.1016/j.acra.2007.09.020. PMC 2475596. PMID 18486005.
- ↑ ZEEMAN, P. (1897). "The Effect of Magnetisation on the Nature of Light Emitted by a Substance". Nature. 55 (1424): 347. Bibcode:1897Natur..55..347Z. doi:10.1038/055347a0. ISSN 0028-0836.
- ↑ Steck, D. A., Rubidium 87 D Line Data. Oregon Center for Optics and Department of Physics, University of Oregon, 2015.
- ↑ Levitt, M. H., Spin Dynamics. John Wiley & Sons, Ltd.: 2003.
- ↑ Dreiling, J. M.; Norrgard, E. B.; Tupa, D.; Gay, T. J. (2012-11-26). "Transverse measurements of polarization in optically pumped Rb vapor cells". Physical Review A. 86 (5): 053416. Bibcode:2012PhRvA..86e3416D. doi:10.1103/physreva.86.053416. ISSN 1050-2947.
- 1 2 3 4 Anger, B. C.; Schrank, G.; Schoeck, A.; Butler, K. A.; Solum, M. S.; Pugmire, R. J.; Saam, B. (2008-10-08). "Gas-phase spin relaxation ofXe129". Physical Review A. 78 (4): 043406. Bibcode:2008PhRvA..78d3406A. doi:10.1103/physreva.78.043406. ISSN 1050-2947.
- 1 2 Chann, B.; Nelson, I. A.; Anderson, L. W.; Driehuys, B.; Walker, T. G. (2002-02-28). "129Xe−Xe Molecular Spin Relaxation". Physical Review Letters. 88 (11): 113201. Bibcode:2002PhRvL..88k3201C. doi:10.1103/physrevlett.88.113201. ISSN 0031-9007. PMID 11909399.
- ↑ Whiting, Nicholas; Eschmann, Neil A.; Goodson, Boyd M.; Barlow, Michael J. (2011-05-26). "Xe129-Cs (D1,D2) versusXe129-Rb (D1) spin-exchange optical pumping at high xenon densities using high-power laser diode arrays". Physical Review A. 83 (5): 053428. Bibcode:2011PhRvA..83e3428W. doi:10.1103/physreva.83.053428. ISSN 1050-2947.
- 1 2 Burant, A. Characterizing Hyperpolarized 129Xe Depolarization Mechanisms during Continuous-Flow Spin Exchange Optical Pumping and as a Source of Image Contrast. University of North Carolina, Chapel Hill, 2018.
- 1 2 Hughes-Riley, Theodore; Six, Joseph S.; Lilburn, David M.L.; Stupic, Karl F.; Dorkes, Alan C.; Shaw, Dominick E.; Pavlovskaya, Galina E.; Meersmann, Thomas (2013). "Cryogenics free production of hyperpolarized 129Xe and 83Kr for biomedical MRI applications". Journal of Magnetic Resonance. 237: 23–33. Bibcode:2013JMagR.237...23H. doi:10.1016/j.jmr.2013.09.008. ISSN 1090-7807. PMC 3863958. PMID 24135800.
- 1 2 3 4 Breeze, Steven R.; Lang, Stephen; Moudrakovski, Igor; Ratcliffe, Chris I.; Ripmeester, John A.; Santyr, Giles; Simard, Benoit; Zuger, Irene (2000). "Coatings for optical pumping cells and short-term storage of hyperpolarized xenon". Journal of Applied Physics. 87 (11): 8013–8017. Bibcode:2000JAP....87.8013B. doi:10.1063/1.373489. ISSN 0021-8979.
- ↑ Sharma, M.; Babcock, E.; Andersen, K. H.; Barrón-Palos, L.; Becker, M.; Boag, S.; Chen, W. C.; Chupp, T. E.; Danagoulian, A. (2008-08-20). "Neutron Beam Effects on Spin-Exchange-PolarizedHe3". Physical Review Letters. 101 (8): 083002. arXiv:0802.3169. Bibcode:2008PhRvL.101h3002S. doi:10.1103/physrevlett.101.083002. ISSN 0031-9007. PMID 18764610. S2CID 29364905.
- 1 2 3 4 5 6 7 8 9 10 Ishikawa, K.; Patton, B.; Jau, Y. -Y.; Happer, W. (2007-05-04). "Spin Transfer from an Optically Pumped Alkali Vapor to a Solid". Physical Review Letters. 98 (18): 183004. Bibcode:2007PhRvL..98r3004I. doi:10.1103/physrevlett.98.183004. ISSN 0031-9007. PMID 17501572.
- 1 2 3 4 5 6 7 8 9 Nikolaou, P.; Coffey, A. M.; Walkup, L. L.; Gust, B. M.; Whiting, N.; Newton, H.; Barcus, S.; Muradyan, I.; Dabaghyan, M. (2013-08-14). "Near-unity nuclear polarization with an open-source 129Xe hyperpolarizer for NMR and MRI". Proceedings of the National Academy of Sciences. 110 (35): 14150–14155. Bibcode:2013PNAS..11014150N. doi:10.1073/pnas.1306586110. ISSN 0027-8424. PMC 3761567. PMID 23946420.
- 1 2 Nikolaou, Panayiotis; Coffey, Aaron M.; Walkup, Laura L.; Gust, Brogan M.; LaPierre, Cristen D.; Koehnemann, Edward; Barlow, Michael J.; Rosen, Matthew S.; Goodson, Boyd M. (2014-01-21). "A 3D-Printed High Power Nuclear Spin Polarizer". Journal of the American Chemical Society. 136 (4): 1636–1642. doi:10.1021/ja412093d. ISSN 0002-7863. PMC 4287367. PMID 24400919.
- 1 2 Ghosh, Rajat K.; Romalis, Michael V. (2010-04-26). "Measurement of spin-exchange and relaxation parameters for polarizingNe21with K and Rb". Physical Review A. 81 (4): 043415. Bibcode:2010PhRvA..81d3415G. doi:10.1103/physreva.81.043415. ISSN 1050-2947. S2CID 4288279.
- ↑ Garg, A., Classical Electromagnetism in a Nutshell. Princeton University Press: 2012.
- ↑ Chen, W. C.; Gentile, T. R.; Ye, Q.; Walker, T. G.; Babcock, E. (2014-07-07). "On the limits of spin-exchange optical pumping of 3He". Journal of Applied Physics. 116 (1): 014903. Bibcode:2014JAP...116a4903C. doi:10.1063/1.4886583. ISSN 0021-8979. S2CID 119764802.
- ↑ Khan; Harvey; Birchall; Irwin; Nikolaou; Schrank; Emami; Dummer; Barlow; Goodson; Chekmenev (2021-10-04). "Enabling Clinical Technologies for Hyperpolarized 129 Xenon Magnetic Resonance Imaging and Spectroscopy". Angewandte Chemie International Edition in English. 60 (41): 22126–22147. doi:10.1002/anie.202015200. ISSN 1521-3773. PMC 8478785. PMID 34018297.
- 1 2 3 4 Kyle, S.; A, T. J., What is Biomarkers. US National Library of Medicine National Institutes of Health 2011, 1.
- ↑ Jeong, Keunhong; Netirojjanakul, Chawita; Munch, Henrik K.; Sun, Jinny; Finbloom, Joel A.; Wemmer, David E.; Pines, Alexander; Francis, Matthew B. (2016). "Targeted Molecular Imaging of Cancer Cells Using MS2-Based 129Xe NMR". Bioconjugate Chemistry. 27 (8): 1796–1801. doi:10.1021/acs.bioconjchem.6b00275. ISSN 1043-1802. PMID 27454679.
- ↑ Chatterjee, Sabarni K; Zetter, Bruce R (2005). "Cancer biomarkers: knowing the present and predicting the future". Future Oncology. 1 (1): 37–50. doi:10.1517/14796694.1.1.37. ISSN 1479-6694. PMID 16555974.
- ↑ Rao, Madhwesha; Stewart, Neil J.; Norquay, Graham; Griffiths, Paul D.; Wild, Jim M. (2016). "High resolution spectroscopy and chemical shift imaging of hyperpolarized 129Xe dissolved in the human brain in vivo at 1.5 tesla". Magnetic Resonance in Medicine. 75 (6): 2227–2234. doi:10.1002/mrm.26241. ISSN 1522-2594. PMC 4950000. PMID 27080441.
- ↑ Rao, Madhwesha R.; Stewart, Neil J.; Griffiths, Paul D.; Norquay, Graham; Wild, Jim M. (2017-08-31). "Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging". Radiology. 286 (2): 659–665. doi:10.1148/radiol.2017162881. ISSN 0033-8419. PMID 28858563.
- 1 2 3 4 5 6 7 8 9 10 11 Pavlovskaya, G. E.; Cleveland, Z. I.; Stupic, K. F.; Basaraba, R. J.; Meersmann, T. (2005-12-12). "Hyperpolarized krypton-83 as a contrast agent for magnetic resonance imaging". Proceedings of the National Academy of Sciences. 102 (51): 18275–18279. Bibcode:2005PNAS..10218275P. doi:10.1073/pnas.0509419102. ISSN 0027-8424. PMC 1317982. PMID 16344474.
- ↑ Barskiy, Danila A.; Coffey, Aaron M.; Nikolaou, Panayiotis; Mikhaylov, Dmitry M.; Goodson, Boyd M.; Branca, Rosa T.; Lu, George J.; Shapiro, Mikhail G.; Telkki, Ville-Veikko (2016-12-05). "NMR Hyperpolarization Techniques of Gases". Chemistry - A European Journal. 23 (4): 725–751. doi:10.1002/chem.201603884. ISSN 0947-6539. PMC 5462469. PMID 27711999.
- ↑ Qing, Kun; Tustison, Nicholas J.; Mugler, John P; Mata, Jaime F.; Lin, Zixuan; Zhao, Li; Wang, Da; Feng, Xue; Shin, Ji Young; Callahan, Sean J.; Bergman, Michael P. (March 2019). "Probing changes in lung physiology in COPD using CT, perfusion MRI and hyperpolarized xenon-129 MRI". Academic Radiology. 26 (3): 326–334. doi:10.1016/j.acra.2018.05.025. ISSN 1076-6332. PMC 6361721. PMID 30087065.
- ↑ Marshall, Helen; Stewart, Neil J.; Chan, Ho-Fung; Rao, Madhwesha; Norquay, Graham; Wild, Jim M. (2021-02-01). "In vivo methods and applications of xenon-129 magnetic resonance". Progress in Nuclear Magnetic Resonance Spectroscopy. 122: 42–62. doi:10.1016/j.pnmrs.2020.11.002. ISSN 0079-6565. PMC 7933823. PMID 33632417.
- ↑ Nikolaou, Panayiotis; Coffey, Aaron M.; Barlow, Michael J.; Rosen, Matthew S.; Goodson, Boyd M.; Chekmenev, Eduard Y. (2014-07-10). "Temperature-Ramped 129Xe Spin-Exchange Optical Pumping". Analytical Chemistry. 86 (16): 8206–8212. doi:10.1021/ac501537w. ISSN 0003-2700. PMC 4139178. PMID 25008290.
- 1 2 3 4 5 Nikolaou, Panayiotis; Coffey, Aaron M.; Ranta, Kaili; Walkup, Laura L.; Gust, Brogan M.; Barlow, Michael J.; Rosen, Matthew S.; Goodson, Boyd M.; Chekmenev, Eduard Y. (2014-04-25). "Multidimensional Mapping of Spin-Exchange Optical Pumping in Clinical-Scale Batch-Mode 129Xe Hyperpolarizers". The Journal of Physical Chemistry B. 118 (18): 4809–4816. doi:10.1021/jp501493k. ISSN 1520-6106. PMC 4055050. PMID 24731261.
- 1 2 3 Ishikawa, Kiyoshi (2011-07-07). "Glass-wool study of laser-induced spin currents en route to hyperpolarized Cs salt". Physical Review A. 84 (1): 013403. Bibcode:2011PhRvA..84a3403I. doi:10.1103/physreva.84.013403. ISSN 1050-2947.
- ↑ Jan H. Ardenkjær-Larsen; Björn Fridlund; Andreas Gram; Georg Hansson; Lennart Hansson; Mathilde H. Lerche; Rolf Servin; Mikkel Thaning; Klaes Golman (2003). "Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR". Proc. Natl. Acad. Sci. U.S.A. 100 (18): 10158–10163. Bibcode:2003PNAS..10010158A. doi:10.1073/pnas.1733835100. PMC 193532. PMID 12930897.
- ↑ Klaes Golman; Jan H. Ardenkjær-Larsen; J. Stefan Petersson; Sven Månsson; Ib Leunbach (2003). "Molecular imaging with endogenous substances". Proc. Natl. Acad. Sci. U.S.A. 100 (18): 10435–10439. Bibcode:2003PNAS..10010435G. doi:10.1073/pnas.1733836100. PMC 193579. PMID 12930896.
- ↑ Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM (2007). "Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy". Nat. Med. 13 (11): 1382–1387. doi:10.1038/nm1650. PMID 17965722. S2CID 11576068.
- ↑ Haukur Jóhannesson; Sven Macholl; Jan H. Ardenkjær-Larsen (2009). "Dynamic Nuclear Polarization of [1-13C]pyruvic acid at 4.6 tesla". J. Magn. Reson. 197 (2): 167–175. Bibcode:2009JMagR.197..167J. doi:10.1016/j.jmr.2008.12.016. PMID 19162518.
- ↑ Natterer, Johannes; Bargon, Joachim (1997). "Parahydrogen induced polarization". Progress in Nuclear Magnetic Resonance Spectroscopy. 31 (4): 293–315. doi:10.1016/s0079-6565(97)00007-1.
- ↑ Duckett, S. B.; Mewis, R. E. (2012). "Application of Parahydrogen Induced Polarization Techniques in NMR Spectroscopy and Imaging". Acc. Chem. Res. 45 (8): 1247–57. doi:10.1021/ar2003094. PMID 22452702.
- ↑ Eshuis, Nan; Aspers, Ruud L.E.G.; van Weerdenburg, Bram J.A.; Feiters, Martin C.; Rutjes, Floris P.J.T.; Wijmenga, Sybren S.; Tessari, Marco (2016). "Determination of long-range scalar 1 H– 1 H coupling constants responsible for polarization transfer in SABRE". Journal of Magnetic Resonance. 265: 59–66. Bibcode:2016JMagR.265...59E. doi:10.1016/j.jmr.2016.01.012. hdl:2066/161984. ISSN 1090-7807. PMID 26859865.
- ↑ Iali, Wissam; Rayner, Peter J.; Duckett, Simon B. (2018). "Using para hydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates". Science Advances. 4 (1): eaao6250. doi:10.1126/sciadv.aao6250. ISSN 2375-2548. PMC 5756661. PMID 29326984.