 | |
| Names | |
|---|---|
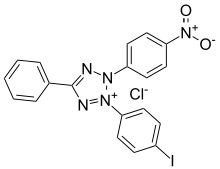
| IUPAC name
3-(4-Iodophenyl)-2-(4-nitrophenyl)-5-phenyl-2H-tetrazol-3-ium chloride | |
| Other names
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.161 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H13ClIN5O2 | |
| Molar mass | 505.70 g·mol−1 |
| Melting point | 240 °C (464 °F; 513 K) (decomposes) |
| Solubility in methanol: water (1:1) | 50 mg/mL hot, very faintly turbid, very deep yellow |
| log P | −2.4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
INT (iodonitrotetrazolium or 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium) is a commonly used tetrazolium salt (usually prepared with chloride ions), similar to tetrazolium chloride that on reduction produces a red formazan dye that can be used for quantitative redox assays. It is also toxic to prokaryotes.[1]
INT is an artificial electron acceptor which can be utilized in a colorimetric assay to determine the concentration of protein in a solution. It can be reduced by succinate dehydrogenase to furazan, the formation of which can be measured by absorbance at 490 nm. The activity of succinate dehydrogenase is readily observed by the naked eye as the solution turns from colorless to rusty red.
See also
References
- ↑ Villegas-Mendoza, Josué; Cajal-Medrano, Ramón; Maske, Helmut (2015). "INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) Is Toxic to Prokaryote Cells Precluding Its Use with Whole Cells as a Proxy for In Vivo Respiration". Microbial Ecology. 70 (4): 1004–1011. doi:10.1007/s00248-015-0626-3. PMID 25991603. S2CID 15264318.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.