 | |
| Names | |
|---|---|
| IUPAC name
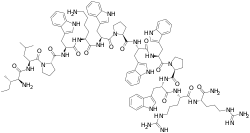
(2S)-1-[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S,3S)-2-amino-3-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]hexanoyl]amino]-3-(1H-indol-3-yl)propanoyl]-N-[(2S)-1-[[(2S)-1-[(2S)-2-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-5-carbamimidamido-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]pyrrolidine-2-carboxamide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C100H132N26O13 | |
| Molar mass | 1906.325 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Indolicidin is an antimicrobial peptide isolated from neutrophil blood cells of cows. The mature peptide is just 13 amino acids, making it one of the smallest antimicrobial peptides known to be encoded as the primary product of the encoding antimicrobial peptide gene.[1] Indolicidin is active against bacterial pathogens, but has also been shown to kill fungi and even HIV virus.[2]
References
- ↑ Selsted, M. E.; Novotny, M. J.; Morris, W. L.; Tang, Y. Q.; Smith, W.; Cullor, J. S. (1992-03-05). "Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils". The Journal of Biological Chemistry. 267 (7): 4292–4295. doi:10.1016/S0021-9258(18)42830-X. ISSN 0021-9258. PMID 1537821.
- ↑ Hancock, Robert E. W.; Scott, Monisha G. (2000-08-01). "The role of antimicrobial peptides in animal defenses". Proceedings of the National Academy of Sciences. 97 (16): 8856–8861. Bibcode:2000PNAS...97.8856H. doi:10.1073/pnas.97.16.8856. ISSN 0027-8424. PMC 34023. PMID 10922046.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.