
An intumescent is a substance that swells as a result of heat exposure, leading to an increase in volume and decrease in density. Intumescence refers to the process of swelling.[1] Intumescent materials are typically used in passive fire protection and require listing, approval, and compliance in their installed configurations in order to comply with the national building codes and laws.
The details for individual building parts are specified in technical standards which are compiled and published by national or international standardization bodies like the British Standards Institute (BSI), the German Institute for Standardization (DIN), the American Society for Testing and Materials (ASTM) or the International Organization for Standardization (ISO).
Intumescent coatings for steel constructions must be approved in standardized fire tests.
Types
Soft char
These intumescent materials produce a light char which is a poor conductor of heat, thus retarding heat transfer. Typically the light char consist of microporous carbonaceous foam formed by a chemical reaction of three main components: ammonium polyphosphate, pentaerythritol, and melamine.[2] The reaction takes place in a matrix formed by the molten binder which is typically based on vinyl acetate copolymers or styrene acrylates.
Ablative coatings contain a significant amount of hydrates. When the hydrates are heated, they decompose, and water vapour is released, which has a cooling effect. Once the water is spent, the insulation characteristics of the char that remains can retard heat transfer through the fire stop assembly.
Soft char products are typically used in thin film intumescent materials for fireproofing protection of structural steel as well as in firestop pillows.
Hard char
Harder char is produced with sodium silicates and graphite. These products are suitable for use in plastic pipe firestops in which applications it is necessary to exert expansion pressure to fill the gap left in the middle of the fire stop assembly left by the melting plastic pipe.
Intumescent coatings
Intumescent coatings may be designed for protection of metals from fire, such as structural steel. Reviews of the technology are available.[3] They may be based on a number of resin binders including epoxy, and silicone.[4] Melamine-formaldehyde resin systems have been used using layered double-hydroxide modified phosphate esters that improved the intumescent properties.[5]
Problems
Some intumescent materials are susceptible to environmental influences such as humidity, which can reduce or negate their ability to function.
Gallery
 Low pressure intumescent resin: This product is suitable for use in passive fire protection in general, and in firestopping and interior fireproofing in particular. The small, orange chunk on the bottom right is capable of growing into the large black object above and to its left.
Low pressure intumescent resin: This product is suitable for use in passive fire protection in general, and in firestopping and interior fireproofing in particular. The small, orange chunk on the bottom right is capable of growing into the large black object above and to its left. Pipe covered with a thin-film intumescent spray fireproofing
Pipe covered with a thin-film intumescent spray fireproofing In this picture, the flame has been removed after the thin-film intumescent spray fireproofing product has completely expanded.
In this picture, the flame has been removed after the thin-film intumescent spray fireproofing product has completely expanded.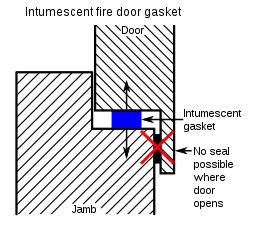 Intumescent gasketing used in passive fire protection, for fire door applications.
Intumescent gasketing used in passive fire protection, for fire door applications.
See also
References
- ↑ Merriam-Webster Dictionary, intumescence, accessed 13 March 2023
- ↑ Werle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3527306732.
- ↑ Puri, Ravindra G.; Khanna, A. S. (2017-01-01). "Intumescent coatings: A review on recent progress". Journal of Coatings Technology and Research. 14 (1): 1–20. doi:10.1007/s11998-016-9815-3. ISSN 1935-3804. S2CID 138961125.
- ↑ Cardoso, de Sa, Beraldo, Hidalgo and Ferreira (November 2020). "Intumescent coatings using epoxy, alkyd, acrylic, silicone and silicone-epoxy hybrid resins for steel fire protection". Journal of Coatings Technology and Research. 17 (6): 1471–1488. doi:10.1007/s11998-020-00366-9. S2CID 220375908.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Xu, Deng and Lang (January 2020). "Flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings reinforced with layered double hydroxides". Journal of Coatings Technology and Research. 17 (1): 157–169. doi:10.1007/s11998-019-00249-8. S2CID 199473315.
External links
- "The proof is in the fire" Chemical Innovation Magazine, American Chemical Society
- American Chemical Society: Fire Retardancy of Polypropylene Composites Using Intumescent Coatings
- ASTM E 2786 - 2010 Standard Test Methods for Measuring Expansion of Intumescent Materials Used in Firestop and Joint Systems
.jpg.webp)