 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropyl formate | |
| Other names
Isobutyl formate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.017 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O2 | |
| Molar mass | 102.133 g·mol−1 |
| Density | 0.885 g/mL |
| Melting point | −96 °C (−141 °F; 177 K) |
| Boiling point | 98.4 °C (209.1 °F; 371.5 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 10 °C (50 °F; 283 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
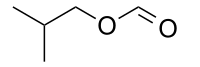
Isobutyl formate (2-methylpropyl methanoate) is an organic ester with the chemical formula C5H10O2. It is formed by the Fischer esterification of isobutanol with formic acid, with the aid of an acid catalyst. It is used as a flavor and fragrance ingredient because of its odor which is sweet, ethereal, and slightly fruity.[2][3]
References
- ↑ Isobutyl formate at Sigma-Aldrich
- ↑ Isobutyl formate, The Good Scents Company
- ↑ Mosciano, Gerard; Sadural, S; Fasano, M; Michalski, J (1989). "Les propriétés organoleptiques des substances aromatisantes" [Organoleptic characteristics of flavor materials]. Perfumer & Flavorist. 14 (6): 47–55.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.