 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.680 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
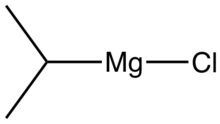
| C3H7ClMg | |
| Molar mass | 102.84 g·mol−1 |
| Solubility | Ethyl ether |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H260, H314 | |
| P210, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P402+P404, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isopropylmagnesium chloride is an organometallic compound with the general formula (CH3)2HCMgCl. This highly flammable, colorless, and moisture sensitive material is the Grignard reagent derived from isopropyl chloride. It is commercially available, usually as a solution in tetrahydrofuran.
Synthesis
Solutions of isopropylmagnesium chloride can be formed by refluxing isopropyl chloride with magnesium metal in ethereal solvents:[1]
(CH3)2HCCl + Mg → (CH3)2HCMgCl
Reactivity
This reagent is used to prepare Grignard reagents by transmetalation reactions as well as installing isopropyl groups.[2] An illustrative generic reaction involves the generation of the Grignard reagent derived from bromo-3,5-bis(trifluoromethyl)benzene:[3]
- (CH3)2HCMgCl + (CF3)2C6H3Br → (CH3)2HCCl + (CF3)2C6H3MgBr
Addition of one equivalent of LiCl to isopropylmagnesium chloride results in the formation of "Turbo Grignard" solutions, named so due to the increased rate and efficiency for transmetallation reactions.[4][5]
Isopropylmagnesium chloride is also used to prepare other isopropyl compounds, such as chlorodiisopropylphosphine:[6]
- PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2
This reaction exploits the bulky nature of the isopropyl substituent.
References
- ↑ Seyferth, Dietmar (2009-03-23). "The Grignard Reagents". Organometallics. 28 (6): 1598–1605. doi:10.1021/om900088z. ISSN 0276-7333.
- ↑ Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. (2003). "Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange". Angewandte Chemie International Edition. 42 (36): 4302–4320. doi:10.1002/anie.200300579. PMID 14502700.
- ↑ Johnnie L. Leazer Jr; Raymond Cvetovich (2005). "A Practical and Safe Preparation of 3,5-Bis(trifluoromethyl)acetophenone". Org. Synth. 82: 115. doi:10.15227/orgsyn.082.0115.
- ↑ Krasovskiy, Arkady; Knochel, Paul (2004-06-21). "A LiCl‐Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl‐ and Heteroarylmagnesium Compounds from Organic Bromides". Angewandte Chemie International Edition. 43 (25): 3333–3336. doi:10.1002/anie.200454084. ISSN 1433-7851.
- ↑ Hermann, Andreas; Seymen, Rana; Brieger, Lukas; Kleinheider, Johannes; Grabe, Bastian; Hiller, Wolf; Strohmann, Carsten (2023-06-19). "Comprehensive Study of the Enhanced Reactivity of Turbo‐Grignard Reagents**". Angewandte Chemie International Edition. 62 (25). doi:10.1002/anie.202302489. ISSN 1433-7851.
- ↑ W. Voskuil; J. F. Arens (1968). "Chlorodiisopropylphosphine". Org. Synth. 48: 47. doi:10.15227/orgsyn.048.0047.