 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methylidenebutanedioic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.364 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6O4 | |
| Molar mass | 130.099 g·mol−1 |
| Appearance | White solid |
| Density | 1.63 g/cm3[1] |
| Melting point | 162 to 164 °C (324 to 327 °F; 435 to 437 K) (decomposes)[1] |
| 1 g/12 mL[1] | |
| Solubility in ethanol | 1 g/5 mL[1] |
| -57.57·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
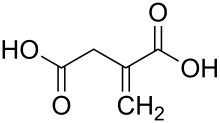
Itaconic acid, or methylidenesuccinic acid, is an organic compound. This dicarboxylic acid is a white solid that is soluble in water, ethanol, and acetone. Historically, itaconic acid was obtained by the distillation of citric acid, but currently it is produced by fermentation. The name itaconic acid was devised as an anagram of aconitic acid, another derivative of citric acid.
Production
Since the 1960s, it is produced industrially by the fermentation of carbohydrates such as glucose or molasses using fungi such as Aspergillus itaconicus or Aspergillus terreus.[2]
For A. terreus the itaconate pathway is mostly elucidated. The generally accepted route for itaconate is via glycolysis, tricarboxylic acid cycle, and a decarboxylation of cis-aconitate to itaconate via cis-aconitate-decarboxylase.[3]
The smut fungus Ustilago maydis uses an alternative route. Cis-aconitate is converted to the thermodynamically favoured trans-aconitate via aconitate-Δ-isomerase (Adi1).[4] trans-Aconitate is further decarboxylated to itaconate by trans-aconitate-decarboxylase (Tad1).[4]
Itaconic acid is also produced in cells of macrophage lineage.[5] It was shown that itaconate is a covalent inhibitor of the enzyme isocitrate lyase in vitro.[6][7] As such, itaconate may possess antibacterial activities against bacteria expressing isocitrate lyase (such as Salmonella enterica and Mycobacterium tuberculosis).[8][9]
However, cells of macrophage lineage have to "pay the price" for making itaconate, and they lose the ability to perform mitochondrial substrate-level phosphorylation.[10]
Laboratory synthesis
Dry distillation of citric acid affords itaconic anhydride, which undergoes hydrolysis to itaconic acid.[11]
Reactions
Upon heating, itaconic anhydride isomerizes to citraconic acid anhydride, which can be hydrolyzed to citraconic acid (2-methylmaleic acid).[12]
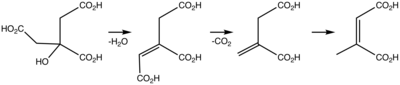
Partial hydrogenation of itaconic acid over Raney nickel affords 2-methylsuccinic acid.[13]
Itaconic acid is primarily used as a co-monomer in the production of acrylonitrile butadiene styrene and acrylate latexes with applications in the paper and architectural coating industry.
References
- 1 2 3 4 5 Merck Index, 11th Edition, 5130
- ↑ Roger A. Sheldon (2014). "Green and sustainable manufacture of chemicals from biomass: state of the art". Green Chem. 16 (3): 950–963. doi:10.1039/C3GC41935E.
- ↑ Steiger, Matthias Georg; Blumhoff, Marzena Lidia; Mattanovich, Diethard; Sauer, Michael (2013-01-01). "Biochemistry of microbial itaconic acid production". Frontiers in Microbiology. 4: 23. doi:10.3389/fmicb.2013.00023. PMC 3572532. PMID 23420787.
- 1 2 Geiser, Elena; Przybilla, Sandra K; Friedrich, Alexandra; Buckel, Wolfgang; Wierckx, Nick; Blank, Lars M; Bölker, Michael (2016-01-01). "Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate". Microbial Biotechnology. 9 (1): 116–126. doi:10.1111/1751-7915.12329. ISSN 1751-7915. PMC 4720413. PMID 26639528.
- ↑ O'Neill, Luke A J; Artyomov, Maxim N (2019). "Itaconate: the poster child of metabolic reprogramming in macrophage function". Nat. Rev. Immunol. 19 (5): 273–281. doi:10.1038/s41577-019-0128-5. PMID 30705422. S2CID 59524706.
- ↑ Kwai, B. X. C.; Collins, A. J.; Middleditch, M. J.; Sperry, J.; Bashiri, G.; Leung, I. K. H. (2020). "Itaconate is a covalent inhibitor of the Mycobacterium tuberculosis isocitrate lyase". RSC Med. Chem. 12 (1): 57–61. doi:10.1039/D0MD00301H. PMC 8130629. PMID 34046597.
- ↑ Rittenhouse, Judith Williams; McFadden, Bruce A (1974). "Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate". Archives of Biochemistry and Biophysics. 163 (1): 79–86. doi:10.1016/0003-9861(74)90456-1. PMID 4852477.
- ↑ Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; Buttini, M.; Linster, C. L.; Medina, E.; Balling, R.; Hiller, K. (2013). "Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production". Proceedings of the National Academy of Sciences. 110 (19): 7820–5. Bibcode:2013PNAS..110.7820M. doi:10.1073/pnas.1218599110. PMC 3651434. PMID 23610393.
- ↑ Cordes, Thekla; Michelucci, Alessandro; Hiller, Karsten (2015). "Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite". Annual Review of Nutrition. 35: 451–73. doi:10.1146/annurev-nutr-071714-034243. PMID 25974697.
- ↑ Nemeth, B.; Doczi, J.; Csete, D.; Kacso, G.; Ravasz, D.; Adams, D.; Kiss, G.; Nagy, A. M.; Horvath, G.; Tretter, L.; Mocsai, A.; Csepanyi-Komi, R.; Iordanov, I.; Adam-Vizi, V.; Chinopoulos, C. (2015). "Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage". The FASEB Journal. 30 (1): 286–300. doi:10.1096/fj.15-279398. PMID 26358042.
- ↑ R. L. Shriner; S. G. Ford; l. J. Roll (1931). "Itaconic anhydride and itaconic acid". Org. Synth. 11: 70. doi:10.15227/orgsyn.011.0070.
- ↑ R. L. Shriner; S. G. Ford; l. J. Roll (1931). "Citraconic Anhydride and Citraconic Acid". Org. Synth. 28: 28. doi:10.15227/orgsyn.011.0028.
- ↑ R. F. Feldkamp; B. F. Tullar (1954). "3-Methylthiophene". Org. Synth. 34: 73. doi:10.15227/orgsyn.034.0073.