 | |
 | |
| Names | |
|---|---|
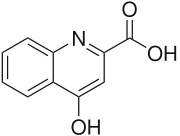
| Preferred IUPAC name
4-Hydroxyquinoline-2-carboxylic acid | |
| Other names
Kinurenic acid, kynuronic acid, quinurenic acid, transtorine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.047 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H7NO3 | |
| Molar mass | 189.168 g/mol |
| Melting point | 282.5 °C (540.5 °F; 555.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Kynurenic acid (KYNA or KYN) is a product of the normal metabolism of amino acid L-tryptophan. It has been shown that kynurenic acid possesses neuroactive activity. It acts as an antiexcitotoxic and anticonvulsant, most likely through acting as an antagonist at excitatory amino acid receptors. Because of this activity, it may influence important neurophysiological and neuropathological processes. As a result, kynurenic acid has been considered for use in therapy in certain neurobiological disorders. Conversely, increased levels of kynurenic acid have also been linked to certain pathological conditions.
Kynurenic acid was discovered in 1853 by the German chemist Justus von Liebig in dog urine, which it was apparently named after.[1]
It is formed from L-kynurenine in a reaction catalyzed by the enzyme kynurenine—oxoglutarate transaminase.[2]
Mechanism of action
KYNA has been proposed to act on five targets:
- As an antagonist at ionotropic AMPA, NMDA and Kainate glutamate receptors in the concentration range of 0.1-2.5 mM.[3]
- As a noncompetitive antagonist at the glycine site of the NMDA receptor.
- As an antagonist of the α7 nicotinic acetylcholine receptor.[4] However, recently (2011) direct recording of α7 nicotinic acetylcholine receptor currents in adult (noncultured) hippocampal interneurons by the Cooper laboratory [5] validated a 2009 study[6] that failed to find any blocking effect of kynurenic acid across a wide range of concentrations, thus suggesting that in noncultured, intact preparations from adult animals there is no effect of kynurenic acid on α7 nicotinic acetylcholine receptor currents.[5][6]
- As a ligand for the orphan G protein-coupled receptor GPR35.[7]
- As an agonist for the G protein-coupled receptor HCAR3.[8]
Role in disease
High levels of kynurenic acid have been identified in patients with tick-borne encephalitis,[9] schizophrenia and HIV-related illnesses. In all these situations, increased levels were associated with confusion and psychotic symptoms. Kynurenic acid acts in the brain as a glycine-site NMDAr antagonist, key in glutamatergic neurotransmission system, which is thought to be involved in the pathophysiology and pathogenesis of schizophrenia.
The kynurenic acid hypothesis of schizophrenia was proposed in 2007,[10][11] based on its action on midbrain dopamine activity and NMDArs, thus linking dopamine hypothesis of schizophrenia with the glutamate hypothesis of the disease.
Kynurenic acid is reduced in individuals with mood disorders, such as major depressive disorder[12] and bipolar disorder,[12] especially during depressive episodes.[13]
High levels of kynurenic acid have been identified in human urine in certain metabolic disorders, such as marked pyridoxine deficiency and deficiency/absence of kynureninase.
When researchers decreased the levels of kynurenic acid in the brains of mice, their cognition was shown to improve markedly.[14] However, kynurenic acid also shows neuroprotective properties.[15] Some researchers have posited that the increased levels found in cases of neurological degradation is due to a failed attempt to protect the cells.[16]
Elevated levels of kynurenic acid compared to kynurenine appear to be associated with poorer T cell response and higher mortality in male subjects with COVID-19, suggesting an explanation for the poorer clinical outcomes observed in males than in females.[17]
Link to ketogenic diet
One controlled study kept mice on a ketogenic diet and measured kynurenic acid concentrations in different parts of the brain.[18] It found that the mice on the ketogenic diet had greater kynurenic acid concentrations in the striatum and hippocampus compared to mice on a normal diet, with no significant difference in the cortex.
In response to the studies showing detrimental behaviour following increases in kynurenic acid[14] the authors also note that the diet was generally well tolerated by the animals, with no "gross behavioural abnormalities". They posit that the increases in concentrations found were insufficient to produce behavioural changes seen in those studies.
See also
References
- ↑ Liebig, J., Uber Kynurensäure, Justus Liebigs Ann. Chem., 86: 125-126, 1853.
- ↑ Han, Qian; Cai, Tao; Tagle, Danilo A.; Robinson, Howard; Li, Jianyong (August 2008). "Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II". Bioscience Reports. 28 (4): 205–215. doi:10.1042/BSR20080085. ISSN 0144-8463. PMC 2559858. PMID 18620547.
- ↑ Elmslie KS, Yoshikami D (March 1985). "Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord". Brain Research. 330 (2): 265–72. doi:10.1016/0006-8993(85)90685-7. PMID 2985194. S2CID 24345638.
- ↑ Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (October 2001). "The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications". The Journal of Neuroscience. 21 (19): 7463–73. doi:10.1523/JNEUROSCI.21-19-07463.2001. PMC 6762893. PMID 11567036.
- 1 2 Dobelis P, Varnell A, Staley K, Cooper D (2011). "Nicotinic α7 acetylcholine receptor-mediated currents are not modulated by the tryptophan metabolite kynurenic acid in adult hippocampal interneurons". Nature Precedings. arXiv:1204.1558. doi:10.1038/npre.2011.6277.1.
- 1 2 Mok MH, Fricker AC, Weil A, Kew JN (September 2009). "Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels". Neuropharmacology. 57 (3): 242–9. doi:10.1016/j.neuropharm.2009.06.003. PMID 19523966. S2CID 29874580.
- ↑ Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L (August 2006). "Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35". The Journal of Biological Chemistry. 281 (31): 22021–22028. doi:10.1074/jbc.M603503200. PMID 16754668.
- ↑ Kapolka NJ, Taghon GJ, Rowe JB, Morgan WM, Enten JF, Lambert NA, Isom DG (June 2020). "DCyFIR: a high-throughput CRISPR platform for multiplexed G protein-coupled receptor profiling and ligand discovery". Proceedings of the National Academy of Sciences of the United States of America. 117 (23): 13117–13126. Bibcode:2020PNAS..11713117K. doi:10.1073/pnas.2000430117. PMC 7293659. PMID 32434907.
- ↑ Holtze M, Mickiené A, Atlas A, Lindquist L, Schwieler L (October 2012). "Elevated cerebrospinal fluid kynurenic acid levels in patients with tick-borne encephalitis". Journal of Internal Medicine. 272 (4): 394–401. doi:10.1111/j.1365-2796.2012.02539.x. hdl:10616/44938. PMID 22443218. S2CID 2255818.
- ↑ Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G (September 2007). "The kynurenic acid hypothesis of schizophrenia". Physiology & Behavior. 92 (1–2): 203–9. doi:10.1016/j.physbeh.2007.05.025. PMID 17573079. S2CID 46156877.
- ↑ Erhardt S, Schwieler L, Engberg G (2003). "Kynurenic Acid and Schizophrenia". Developments in Tryptophan and Serotonin Metabolism. Advances in Experimental Medicine and Biology. Vol. 527. pp. 155–65. doi:10.1007/978-1-4615-0135-0_18. ISBN 978-1-4613-4939-6. PMID 15206728.
- 1 2 Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, et al. (November 2020). "The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies". Molecular Psychiatry. 26 (8): 4158–4178. doi:10.1038/s41380-020-00951-9. PMID 33230205. S2CID 227132820.
- ↑ Bartoli F, Misiak B, Callovini T, Cavaleri D, Cioni RM, Crocamo C, et al. (October 2020). "The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites". Molecular Psychiatry. 26 (7): 3419–3429. doi:10.1038/s41380-020-00913-1. PMID 33077852. S2CID 224314102.
- 1 2 Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R (July 2010). "Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior". Neuropsychopharmacology. 35 (8): 1734–42. doi:10.1038/npp.2010.39. PMC 3055476. PMID 20336058.
- ↑ Urbańska EM, Chmiel-Perzyńska I, Perzyński A, Derkacz M, Owe-Larsson B (2014). "Endogenous Kynurenic Acid and Neurotoxicity". Handbook of Neurotoxicity. pp. 421–453. doi:10.1007/978-1-4614-5836-4_92. ISBN 978-1-4614-5835-7.
- ↑ Zádori D, Klivényi P, Vámos E, Fülöp F, Toldi J, Vécsei L (November 2009). "Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies" (PDF). Journal of Neural Transmission. 116 (11): 1403–9. doi:10.1007/s00702-009-0263-4. PMID 19618107. S2CID 11378729.
- ↑ Cai Y, Kim DJ, Takahashi T, Broadhurst DI, Yan H, Ma S, et al. (July 2021). "Kynurenic acid may underlie sex-specific immune responses to COVID-19". Science Signaling. 14 (690): eabf8483. doi:10.1126/scisignal.abf8483. ISSN 1945-0877. PMC 8432948. PMID 34230210.
- ↑ Żarnowski T, Chorągiewicz T, Tulidowicz-Bielak M, Thaler S, Rejdak R, Żarnowski I, et al. (June 2012). "Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats". Journal of Neural Transmission. 119 (6): 679–84. doi:10.1007/s00702-011-0750-2. PMC 3359463. PMID 22200857.
External links
- Link found between TBE and schizophrenia - TheLocal.se, Sweden's news in English, 6 November 2007.