 | |
| Names | |
|---|---|
| IUPAC name
Lead(IV) acetate | |
| Systematic IUPAC name
Tetrakis(acetyloxy)plumbane | |
| Other names
Lead tetraacetate Plumbic acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.099 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Pb(C2H3O2)4 | |
| Molar mass | 443.376 g/mol |
| Appearance | colorless or pink crystals |
| Odor | vinegar |
| Density | 2.228 g/cm3 (17 °C) |
| Melting point | 175 °C (347 °F; 448 K) |
| Boiling point | decomposes |
| soluble, reversible hydrolysis | |
| Solubility | reacts with ethanol soluble in chloroform, benzene, nitrobenzene, hot acetic acid, HCl, tetrachloroethane |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic |
| GHS labelling:[1] | |
   | |
| Danger | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.[2]
Structure
In the solid state the lead(IV) centers are coordinated by four acetate ions, which are bidentate, each coordinating via two oxygen atoms. The lead atom is 8 coordinate and the O atoms form a flattened trigonal dodecahedron.[3]
Preparation
It is typically prepared by treating of red lead with acetic acid and acetic anhydride (Ac2O), which absorbs water. The net reaction is shown:[4]
- Pb3O4 + 4 Ac2O → Pb(OAc)4 + 2 Pb(OAc)2
The remaining lead(II) acetate can be partially oxidized to the tetraacetate:
- 2 Pb(OAc)2 + Cl2 → Pb(OAc)4 + PbCl2
Reagent in organic chemistry
Lead tetraacetate is a strong oxidizing agent,[5] a source of acetyloxy groups and a general reagent for the introduction of lead into organolead compounds. Some of its many uses in organic chemistry:
- Acetoxylation of benzylic, allylic and α-oxygen ether C−H bonds, for example the photochemical conversion of dioxane to 1,4-dioxene through the 2-acetoxy-1,4-dioxane intermediate [6] and the conversion of α-pinene to verbenone[7]
- An alternative reagent to bromine in Hofmann rearrangement[8]
- Oxidation of hydrazones to diazo compounds, for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane[9]
- Aziridine formation, for example the reaction of N-aminophthalimide and stilbene[10]
- Cleavage of α-hydroxy acids[11] or 1,2-diols to their corresponding aldehydes or ketones, often replacing ozonolysis; for instance, the oxidation of di-n-butyl D-tartrate to n-butyl glyoxylate.[12]
- Reaction with alkenes to form γ-lactones
- Oxidation of alcohols carrying a δ-proton to cyclic ethers.[13]
- Oxidative cleavage of certain allyl alcohols in conjunction with ozone:[14][15]
- Transformation of 1,2-dicarboxylic acids or cyclic anhydrides to alkenes
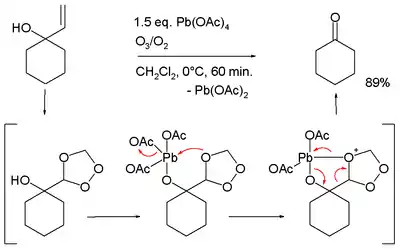
- Conversion of acetophenones to phenyl acetic acids[16]
- Decarboxylation of carboxylic acids to alkyl halides in the Kochi reaction[17]
Safety
Lead(IV) acetate may be fatal if ingested, inhaled, or absorbed through skin. It causes irritation to skin, eyes, and respiratory tract. It is a neurotoxin. It affects the gum tissue, central nervous system, kidneys, blood, and reproductive system.
References
- ↑ "Substance Information - ECHA". echa.europa.eu.
- ↑ Mihailo Lj. Mihailović; Živorad Čeković; Brian M. Mathes (2005). "Lead(IV) Acetate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rl006.pub2. ISBN 978-0-471-93623-7.
- ↑ Schürmann, M.; Huber, F. (1994). "A redetermination of lead(IV) acetate". Acta Crystallographica Section C. 50 (11): 1710–1713. doi:10.1107/S0108270194006438. ISSN 0108-2701.
- ↑ J. C. Bailar, Jr. (1939). "Lead Tetracetate". Inorganic Syntheses. Inorganic Syntheses. Vol. 1. pp. 47–49. doi:10.1002/9780470132326.ch17. ISBN 978-0-470-13232-6.
- ↑ J. Zýka (1966). "Analytical study of the basic properties of lead tetraacetate as oxidizing agent" (PDF). Pure and Applied Chemistry. 13 (4): 569–581. doi:10.1351/pac196613040569. S2CID 96821219. Retrieved 19 December 2013.
- ↑ Organic Syntheses, Vol. 82, p.99 (2005) Article.
- ↑ Organic Syntheses, Coll. Vol. 9, p.745 (1998); Vol. 72, p.57 (1995) Article
- ↑ Baumgarten, Henry; Smith, Howard; Staklis, Andris (1975). "Reactions of amines. XVIII. Oxidative rearrangement of amides with lead tetraacetate". The Journal of Organic Chemistry. 40 (24): 3554–3561. doi:10.1021/jo00912a019.
- ↑ Organic Syntheses, Coll. Vol. 6, p.161 (1988); Vol. 50, p.6 (1970) Article.
- ↑ Organic Syntheses, Coll. Vol. 6, p.56 (1988); Vol. 55, p.114 (1976) Link
- ↑ Ōeda, Haruomi (1934). "Oxidation of some α-hydroxy-acids with lead tetraacetate". Bulletin of the Chemical Society of Japan. 9 (1): 8–14. doi:10.1246/bcsj.9.8.
- ↑ Organic Syntheses, Coll. Vol. 4, p.124 (1963); Vol. 35, p.18 (1955) Article.
- ↑ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN 0-471-58589-0)
- ↑ Álvarez Manzaneda, E. J.; Chahboun, R.; Cano, M. J.; Cabrera Torres, E.; Álvarez, E.; Álvarez Manzaneda, R.; Haidour, A.; Ramos López, J. M. (2006). "O3/Pb(OAc)4: a new and efficient system for the oxidative cleavage of allyl alcohols". Tetrahedron Letters. 47 (37): 6619–6622. doi:10.1016/j.tetlet.2006.07.020.
- ↑ Conversion of 1-allylcyclohexanol to cyclohexanone, in the proposed reaction mechanism the allyl group is first converted to a trioxalane according to conventional ozonolysis which then interacts with the alkoxy lead group.
- ↑ Myrboh, B.; Ila, H.; Junjappa, H. (1981). "One-Step Synthesis of Methyl Arylacetates from Acetophenones Using Lead(IV) Acetate". Synthesis. 2 (2): 126–127. doi:10.1055/s-1981-29358.
- ↑ Jay K. Kochi (1965). "A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate". J. Am. Chem. Soc. 87 (11): 2500–02. doi:10.1021/ja01089a041.
