 | |
| Names | |
|---|---|
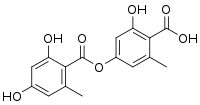
| Preferred IUPAC name
4-[(2,4-Dihydroxy-6-methylbenzoyl)oxy]-2-hydroxy-6-methylbenzoic acid | |
| Other names
Orsellinate depside | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14O7 | |
| Molar mass | 318.281 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lecanoric acid is a chemical produced by several species of lichen.[1] Lecanoric acid is classified as a polyphenol and a didepside, and it functions as an antioxidant.[2] The acid is named after the lichen Lecanora. The acid has also been isolated from Usnea subvacata, Parmotrema stuppuem, Parmotrema tinctorum, Parmotrema grayana, Xanthoparmelia arida and Xanthoparmelia lecanorica.[3][4] A related compound, 5-chlorolecanoric acid, is found in some species of Punctelia.[5]
References
- ↑ "Lecanoric acid". PubChem. National Center for Biotechnology Information. Retrieved 27 June 2019.
- ↑ Luo, Heng; Yamamoto, Yoshikazu; A Kim, Jung; Jung, Jae Sung; Koh, Young Jin; Hur, Jae-Seoun (13 March 2009). "Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island)". Polar Biology. 32 (7): 1033–1040. Bibcode:2009PoBio..32.1033L. doi:10.1007/s00300-009-0602-9. S2CID 9256291.
- ↑ Hale, Mason E. (1990). A synopsis of the lichen genus Xanthoparmelia (Vainio) Hale (Ascomycotina, Parmeliaceae) /. Washington, D.C.: Smithsonian Institution Press. doi:10.5962/bhl.title.123253.
- ↑ White, Pollyanna; Oliveira, Rita; Oliveira, Aldeidia; Serafini, Mairim; Araújo, Adriano; Gelain, Daniel; Moreira, Jose; Almeida, Jackson; Quintans, Jullyana; Quintans-Junior, Lucindo; Santos, Marcio (12 September 2014). "Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review". Molecules. 19 (9): 14496–14527. doi:10.3390/molecules190914496. PMC 6271897. PMID 25221871.
- ↑ Elix, John A.; Wardlaw, Judith H. (2002). "5-Chlorolecanoric acid, a new depside from Punctelia species" (PDF). Australasian Lichenology. 50: 6–9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.