In organic chemistry, the Malaprade reaction or Malaprade oxidation is a glycol cleavage reaction in which a vicinal diol is oxidized by periodic acid or a periodate salt to give the corresponding carbonyl functional groups.[1][2] The reaction was first reported by Léon Malaprade in 1928. Amino alcohols are also cleaved.
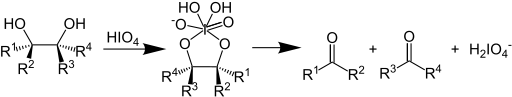
In terms of mechanism, the reaction is thought to proceed by a cyclic diester of iodine(VII).[3]

References
- ↑ Christopher R. Schmid, Jerry D. Bryant (1995). "D-(R)-Glyceraldehyde Acetonide". Organic Syntheses. 72: 6. doi:10.15227/orgsyn.072.0006.
- ↑ "406. Malaprade Reaction (Malaprade Oxidation)". Comprehensive Organic Name Reactions and Reagents. Wiley. 2010. pp. 1807–1810. doi:10.1002/9780470638859.conrr406.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1732-1734, ISBN 978-0-471-72091-1
See also
Historic references
- Dupuis, Gérard. "Les alcools" [Alcohols]. Cours de chimie Organique (in French). Lycée Faidherbe de Lille.
Les α-glycols subissent une réaction de coupure en présence d'acide périodique HIO4 pour donner des composés carbonylés. Il s'agit de la réaction introduite en 1928 par le chimiste français L. Malaprade et qui porte son nom. [In the presence of periodic acid, HIO4, α-glycols react, cleaving into their component carbonyls.]
- L. Malaprade (1928). Bull. Soc. Chim. France. 43: 683.
{{cite journal}}: Missing or empty|title=(help) - Compt. Rend. 186: 382. 1928.
{{cite journal}}: Missing or empty|title=(help) - Nicolet, Ben H.; Shinn, Leo A. (1939). "The Action of Periodic Acid on α-Amino Alcohols". J. Am. Chem. Soc. 61 (6): 1615. doi:10.1021/ja01875a521.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.