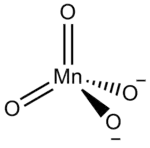
In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom.[1] However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state.[1] Manganates are the only known manganese(VI) compounds.[2]
Other manganates include hypomanganate or manganate(V), MnO3−
4, permanganate or manganate(VII), MnO−
4, and the dimanganate or dimanganate(III) Mn
2O6−
6.
A manganate(IV) anion MnO4−
4 has been prepared by radiolysis of dilute solutions of permanganate.[3][4] It is mononuclear in dilute solution, and shows a strong absorption in the ultraviolet and a weaker absorption at 650 nm.[3]
Structure

The manganate(VI) ion is tetrahedral, similar to sulfate or chromate: indeed, manganates are often isostructural with sulfates and chromates, a fact first noted by Eilhard Mitscherlich in 1831.[5] The manganese–oxygen distance is 165.9 pm, about 3 pm longer than in permanganate.[5] As a d1 ion, it is paramagnetic, but any Jahn–Teller distortion is too small to be detected by X-ray crystallography.[5] Manganates are dark green in colour, with a visible absorption maximum of λmax = 606 nm (ε = 1710 dm3 mol−1 cm−1).[6][7] The Raman spectrum has also been reported.[8]
Preparation
Sodium and potassium manganates are usually prepared in the laboratory by stirring the equivalent permanganate in a concentrated solution (5–10 M) of the hydroxide for 24 hours[6] or with heating.[9]
- 4 MnO−
4 + 4 OH− → 4 MnO2−
4 + 2 H2O + O2
Potassium manganate is prepared industrially, as an intermediate to potassium permanganate, by dissolving manganese dioxide in molten potassium hydroxide with potassium nitrate or air as the oxidizing agent.[2]
- 2 MnO2 + 4 OH− + O2 → 2 MnO2−
4 + 2 H2O
Disproportionation
Manganates are unstable towards disproportionation in all but the most alkaline of aqueous solutions.[2] The ultimate products are permanganate and manganese dioxide, but the kinetics are complex and the mechanism may involve protonated and/or manganese(V) species.[10][11]
Uses
Manganates, particularly the insoluble barium manganate, BaMnO4, have been used as oxidizing agents in organic synthesis: they will oxidize primary alcohols to aldehydes and then to carboxylic acids, and secondary alcohols to ketones.[12][13] Barium manganate has also been used to oxidize hydrazones to diazo compounds.[14]
Related compounds
Manganate is formally the conjugate base of hypothetical manganic acid H
2MnO
4, which cannot be formed because of its rapid disproportionation. However, its second acid dissociation constant has been estimated by pulse radiolysis techniques:[3]
- HMnO−
4 ⇌ MnO2−
4 + H+ pKa = 7.4 ± 0.1
Manganites
The name "manganite" is used for compounds formerly believed to contain the anion MnO3−
3, with manganese in the +3 oxidation state. However, most of these "manganites" do not contain discrete oxyanions, but are mixed oxides with perovskite (LaMnIIIO3, CaMnIVO3), spinel (LiMnIII,IV
2O4) or sodium chloride (LiMnIIIO2, NaMnIIIO2) structures.
One exception is potassium dimanganate(III), K6Mn2O6, which contains discrete Mn2O6−
6 anions.[15]
References
- 1 2 International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 74–75, 77–78, 313, 338. Electronic version..
- 1 2 3 Cotton, F. Albert; Wilkinson, Geoffrey (1980), Advanced Inorganic Chemistry (4th ed.), New York: Wiley, p. 746, ISBN 0-471-02775-8.
- 1 2 3 Rush, J. D.; Bielski, B. H. J. (1995), "Studies of Manganate(V), -(VI), and -(VII) Tetraoxyanions by Pulse Radiolysis. Optical Spectra of Protonated Forms", Inorg. Chem., 34 (23): 5832–38, doi:10.1021/ic00127a022
- ↑ Lee, Donald G.; Chen, Tao (1989), "Oxidation of hydrocarbons. 18. Mechanism of the reaction between permanganate and carbon-carbon double bonds", J. Am. Chem. Soc., 111 (19): 7534–38, doi:10.1021/ja00201a039.
- 1 2 3 Palenik, Gus J. (1967), "Crystal structure of potassium manganate", Inorg. Chem., 6 (3): 507–11, doi:10.1021/ic50049a016.
- 1 2 Carrington, A.; Symons, M. C. R. (1956), "Structure and reactivity of the oxy-anions of transition metals. Part I. The manganese oxy-anions", J. Chem. Soc.: 3373–80, doi:10.1039/JR9560003373
- ↑ Lee, Donald G.; Chen, Tao (1993), "Reduction of manganate(VI) by mandelic acid and its significance for development of a general mechanism of dationoxin of organic compounds by high-valent transition metal oxides", J. Am. Chem. Soc., 115 (24): 11231–36, doi:10.1021/ja00077a023.
- ↑ Juberta, A. H.; Varettia, E. L. (1982), "Normal and resonance Raman spectra of some manganates", J. Mol. Struct., 79 (1–2): 285–88, Bibcode:1982JMoSt..79..285J, doi:10.1016/0022-2860(82)85067-9
- ↑ Nyholm, R. S.; Woolliams, P. R. (1968), "Manganates(VI)", Inorg. Synth., 11: 56–61
- ↑ Sutter, Joan H.; Colquitt, Kevin; Sutter, John R. (1974), "Kinetics of the disproportionation of manganate in acid solution", Inorg. Chem., 13 (6): 1444–46, doi:10.1021/ic50136a037.
- ↑ Sekula-Brzezińska, K.; Wrona, P. K.; Galus, Z. (1979), "Rate of the MnO4−/MnO42− and MnO42−/MnO43− electrode reactions in alkaline solutions at solid electrodes", Electrochim. Acta, 24 (5): 555–63, doi:10.1016/0013-4686(79)85032-X.
- ↑ Procter, G.; Ley, S. V.; Castle, G. H. (2004), "Barium Manganate", in Paquette, L. (ed.), Encyclopedia of Reagents for Organic Synthesis, New York: Wiley, doi:10.1002/047084289X, hdl:10261/236866, ISBN 9780471936237.
- ↑ Firouzabadi, Habib; Mostafavipoor, Zohreh (1983), "Barium Manganate. A Versatile Oxidant in Organic Synthesis", Bull. Chem. Soc. Jpn., 56 (3): 914–17, doi:10.1246/bcsj.56.914.
- ↑ Guziec, Frank S., Jr.; Murphy, Christopher J.; Cullen, Edward R. (1985), "Thermal and photochemical studies of symmetrical and unsymmetrical dihydro-1,3,4-selenadiazoles", J. Chem. Soc., Perkin Trans. 1: 107–13, doi:10.1039/P19850000107
{{citation}}: CS1 maint: multiple names: authors list (link) - ↑ Brachtel, G.; Hoppe, R. (1976), "Das erste Oxomanganat(III) mit Inselstruktur: K6[Mn2O6]", Naturwissenschaften, 63 (7): 339, Bibcode:1976NW.....63..339B, doi:10.1007/BF00597313, S2CID 45344152.