The metallo-ene reaction is a chemical reaction employed within organic synthesis. Mechanistically similar to the classic ene reaction,[1] the metallo-ene reaction involves a six-member cyclic transition state that brings an allylic species and an alkene species together to undergo a rearrangement. The initial allylic group migrates to one terminus of the alkene reactant and a new carbon-carbon sigma bond is formed between the allylic species and the other terminus of the alkene reactant. In the metallo-ene reaction, a metal ion (Mg, Zn, Pd, Ni etc.)[2][3][4] acts as the migrating group rather than a hydrogen atom as in the classic ene reaction.

Initial Studies
Metallo-ene reaction was first studied by Lehmkuhl et al.,[5] and since then has gradually gained popularity among the synthetic community throughout the better understanding of its mechanism and potential as a synthetic tool.[6]
Classification
Generally speaking, metallo-ene reaction has both an intramolecular and an intermolecular version. For the former, the reaction can be classified into two types by the skeletal connectivity.[7] In Type I, a carbon linkage connects the alkene fragment to the terminal carbon of the allyl fragment of the molecule, while in Type II the alkene fragment is connected to the internal carbon of the allyl fragment.

Mechanism
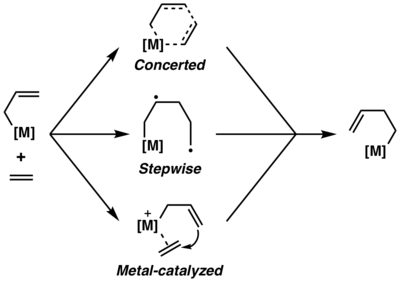
Historically, there has been a long-standing uncertainty about the precise mechanism of metallo-ene reaction.[8] Three possible mechanisms—a concerted mechanism, a stepwise mechanism and a metal-catalyzed mechanism have been postulated and studied over the past few decades. According to computational analyses,[9][10] metallo-ene reaction tends to proceed via a concerted six-member transition state, although the exact mechanism was found to vary and depends on the metal.
Selectivity
Regioselectivity
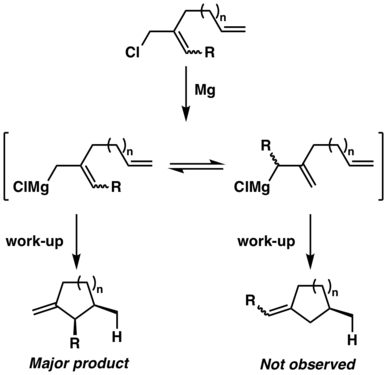
For Type II reaction, two possible products can be expected if the two termini of the allyl piece are unsymmetrically substituted, depending on which carbon engages in the formation of a new sigma bond. Interestingly, Oppolzer et al.[11] have found that the more substituted terminus of the allyl piece will participate in new sigma bond formation regardless of the length of the internal carbon linkage.
Stereoselectivity
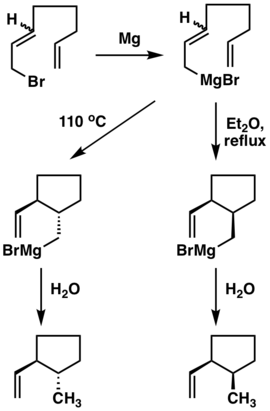
Since a six-member cyclic transition state is involved in metallo-ene reaction, high level of stereoselectivity can be expected due to the conservation of orbital symmetry.[12] Indeed, this happens to be the case in many synthetic applications of this reaction. Felkin et al.[13] have found the cis product to be formed as the predominant product kinetically, while the trans product could also be obtained selectively under thermodynamic conditions. The fact that stereochemical outcome of this metallo-ene reaction could be tuned by altering the reaction conditions regardless of the geometry of allyl fragment reveals its reversible nature.[14]
Synthetic Application
Asymmetric synthesis
In 2016, Trost et al.[15] have developed a highly diastero- and enantioselective intramolecular interrupted metallo-ene reaction using a chiral phosphoramidite ligand to achieve high levels of stereoselectivity. Starting from linear precursors, a wide range of vicinally disubstituted five-member rings could be synthesized. An additional stereocenter is generated during the process by reaction with water.

Sequential coupling
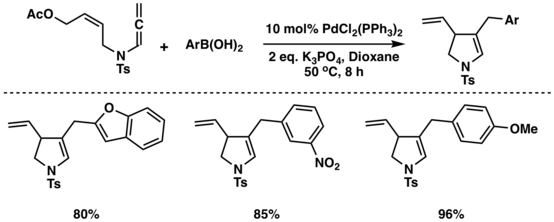
In 2017, Liu et al.[16] have developed a highly efficient palladium- catalyzed cascade metallo-ene/Suzuki coupling reaction of allene-amides, delivering polyfunctionalized 2,3-dihydropyrrole derivatives in excellent yields.
Total synthesis
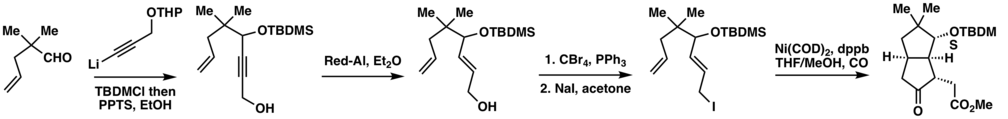
In their synthetic efforts towards Coriolin, Oppolzer et al.[17] devised a metallo-ene-carbonylation cascade reaction to construct the fused bicyclic core of Coriolin in an efficient fashion. They started with a simple aldehyde to which a propargyl alcohol appendage was attached via nucleophilic addition. Reduction followed by Appel reaction and Finkelstein reaction would yielded a key intermediate, which in the presence of nickel catalyst and CO atmosphere could be transformed to the target cyclopentanone in decent yield.
References
- ↑ Hoffmann, H. M. R. (August 1969). "The Ene Reaction". Angewandte Chemie International Edition in English. 8 (8): 556–577. doi:10.1002/anie.196905561. ISSN 0570-0833.
- ↑ HIROI, Kunio; HIRASAWA, Kazushige (1994). "Palladium-Catalyzed Intramolecular Metallo-Ene Reactions Using Allylic Sulfones as Enophiles". Chemical & Pharmaceutical Bulletin. 42 (4): 786–791. doi:10.1248/cpb.42.786. ISSN 0009-2363.
- ↑ Michelet, Véronique; Galland, Jean-Christophe; Charruault, Lise; Savignac, Monique; Genêt, Jean-Pierre (June 2001). "Efficient Metallo−Ene Reactions in Organoaqueous Phase". Organic Letters. 3 (13): 2065–2067. doi:10.1021/ol016023f. ISSN 1523-7060.
- ↑ Korotvička, Aleš; Hybelbauerová, Simona; Kotora, Martin (September 2009). "Synthesis of trans-Fused Sesquiterpenoid Analogues by Zirconocene-Mediated Metallo-ene Reaction". Synlett. 2009 (15): 2445–2448. doi:10.1055/s-0029-1217738. ISSN 0936-5214.
- ↑ Lehmkuhl, H.; Reinehr, D. (December 1970). "Die addition von allylmagnesium-halogeniden an 1-alkene". Journal of Organometallic Chemistry. 25 (2): C47–C50. doi:10.1016/S0022-328X(00)87810-9.
- ↑ Oppolzer, Wolfgang (1991), "Metallo-ene Reactions", Comprehensive Organic Synthesis, Elsevier, pp. 29–61, doi:10.1016/b978-0-08-052349-1.00119-0, ISBN 9780080523491
- ↑ Oppolzer, Wolfgang (January 1989). "Intramolecular, Stoichiometric (Li, Mg, Zn) and Catalytic (Ni, Pd, Pt) Metallo-Ene Reactions in Organic Synthesis [New Synthetic Methods (75)]". Angewandte Chemie International Edition in English. 28 (1): 38–52. doi:10.1002/anie.198900381. ISSN 0570-0833.
- ↑ Dubac, Jacques.; Laporterie, Andre. (April 1987). "Ene and retro-ene reactions in group 14 organometallic chemistry". Chemical Reviews. 87 (2): 319–334. doi:10.1021/cr00078a003. ISSN 0009-2665.
- ↑ Yamada, Tomoyuki; Udagawa, Taro; Sakai, Shogo (2010). "Driving force of metallo (Mg–H and Mg–Cl)-ene reaction mechanisms". Physical Chemistry Chemical Physics. 12 (15): 3799–805. doi:10.1039/b925166a. ISSN 1463-9076. PMID 20358073.
- ↑ Sakai, Shogo; Hikida, Takahiro (2008-10-30). "Theoretical Studies of Metallo (Li and Na)−Ene Reaction Mechanisms". The Journal of Physical Chemistry A. 112 (43): 10985–10992. Bibcode:2008JPCA..11210985S. doi:10.1021/jp806506g. ISSN 1089-5639. PMID 18839936.
- ↑ Oppolzer, Wolfgang; Pitteloud, Rita; Strauss, Heinrich F. (November 1982). "Intramolecular type-II "metallo-ene" reactions of (2-alkenylallyl)magnesium chlorides: regio- and stereochemical studies". Journal of the American Chemical Society. 104 (23): 6476–6477. doi:10.1021/ja00387a067. ISSN 0002-7863.
- ↑ Woodward, R.B.; Hoffmann, R. (1971), The Conservation of Orbital Symmetry, Elsevier, p. 37, doi:10.1016/b978-1-4832-3290-4.50006-4, ISBN 9781483232904
- ↑ Felkin, Hugh; David Umpleby, J.; Hagaman, Edward; Wenkert, Ernest (January 1972). "Duality of mechanism in the addition of allylic grignard reagents to carbon-carbon double bonds. The stereoselective cyclisation of 2,7-octadienylmagnesium bromide to -(2-vinylcyclopentyl)-methylmagnesium bromide". Tetrahedron Letters. 13 (22): 2285–2288. doi:10.1016/S0040-4039(01)84829-2.
- ↑ Felkin, Hugh; Kwart, Lawrence D.; Swierczewski, Gérard; Umpleby, J. David (1975). "Nickel complex-catalysed reaction of propylmagnesium bromide with butadiene. A highly stereoselective synthesis of cis- and trans-(2-vinylcyclopentyl)methylmagnesium bromide". Journal of the Chemical Society, Chemical Communications (7): 242. doi:10.1039/c39750000242. ISSN 0022-4936.
- ↑ Trost, Barry M.; Ryan, Michael C. (2016-03-09). "A Ruthenium/Phosphoramidite-Catalyzed Asymmetric Interrupted Metallo-ene Reaction". Journal of the American Chemical Society. 138 (9): 2981–2984. doi:10.1021/jacs.6b00983. ISSN 0002-7863. PMID 26899551.
- ↑ Liang, Hanbing; Yan, Fachao; Dong, Xu; Liu, Qing; Wei, Xiaobing; Liu, Sheng; Dong, Yunhui; Liu, Hui (2017). "Palladium-catalyzed cascade metallo-ene/Suzuki coupling reaction of allenamides". Chemical Communications. 53 (21): 3138–3141. doi:10.1039/C7CC00191F. ISSN 1359-7345. PMID 28246668.
- ↑ Oppolzer, Wolfgang; Keller, Thomas H.; Kuo, David L.; Pachinger, Werner (January 1990). "Palladium(0)- and nickel(0) catalyzed "metallo-ene-type" cyclizations: Stereodirecting resident chirality". Tetrahedron Letters. 31 (9): 1265–1268. doi:10.1016/S0040-4039(00)88781-X.