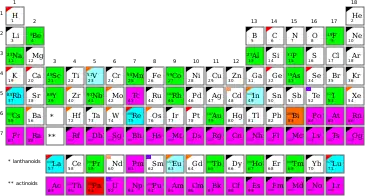
A monoisotopic element is an element which has only a single stable isotope (nuclide). There are 26 such elements, as listed.
Stability is experimentally defined for chemical elements, as there are a number of stable nuclides with atomic numbers over ~ 40 which are theoretically unstable, but apparently have half-lives so long that they have not been observed either directly or indirectly (from measurement of products) to decay.
Monoisotopic elements are characterized, except in one case, by odd numbers of protons (odd Z), and even numbers of neutrons. Because of the energy gain from nuclear pairing, the odd number of protons imparts instability to isotopes of an odd Z, which in heavier elements requires a completely paired set of neutrons to offset this tendency into stability. (The five stable nuclides with odd Z and odd neutron numbers are hydrogen-2, lithium-6, boron-10, nitrogen-14, and tantalum-180m1.)
The single mononuclidic exception to the odd Z rule is beryllium; its single stable, primordial isotope, beryllium-9, has 4 protons and 5 neutrons. This element is prevented from having a stable isotope with equal numbers of neutrons and protons (beryllium-8, with 4 of each) by its instability toward alpha decay, which is favored due to the extremely tight binding of helium-4 nuclei. It is prevented from having a stable isotope with 4 protons and 6 neutrons by the very large mismatch in proton/neutron ratio for such a light element. (Nevertheless, beryllium-10 has a half-life of 1.36 million years, which is too short to be primordial, but still indicates unusual stability for a light isotope with such an imbalance.)
Differentiation from mononuclidic elements
The set of monoisotopic elements overlap but are not the same as the set of 21 mononuclidic elements, which are characterized as having essentially only one isotope (nuclide) found in nature.[1] The reason for this is the occurrence of certain long-lived radioactive primordial nuclides in nature, which may form admixtures with the monoisotopics, and thus prevent them from being naturally mononuclidic. This happens in the cases of 7 of the monoisotopic elements. These isotopes are monoisotopic, but due to the presence of the long-lived radioactive primordial nuclide, are not mononuclidic. These elements are vanadium, rubidium, indium, lanthanum, europium, lutetium and rhenium. See the list below; for (indium and rhenium), the long-lived radionuclide is actually the most abundant isotope in nature, and the stable isotope is less abundant.
In 2 additional cases (bismuth[2] and protactinium), mononuclidic elements occur primordially which are not monoisotopic because the naturally occurring nuclide is radioactive, and thus the element has no stable isotopes at all. For an element to be monoisotopic, it must have one stable nuclide.
List of (observationally-stable) monoisotopic elements, ordered by atomic number and weight
Non-mononuclidic elements are marked with an asterisk, and the long-lived primordial radioisotope given. In two cases (indium and rhenium), the most abundant naturally occurring isotope is the mildly radioactive one, and in the case of europium, nearly half of it is.
- Beryllium-9
- Fluorine-19
- Sodium-23
- Aluminium-27
- Phosphorus-31
- Scandium-45
- Vanadium-51* naturally occurs with 0.25% of radioactive vanadium-50
- Manganese-55
- Cobalt-59
- Arsenic-75
- Rubidium-85* naturally occurs with 27.835% of radioactive rubidium-87
- Yttrium-89
- Niobium-93
- Rhodium-103
- Indium-113* naturally occurs with majority (95.7%) radioactive isotope indium-115
- Iodine-127
- Caesium-133
- Lanthanum-139* naturally occurs with 0.09% radioactive lanthanum-138
- Praseodymium-141
- Europium-153* naturally occurs with 47.8% radioactive europium-151
- Terbium-159
- Holmium-165
- Thulium-169
- Lutetium-175* naturally occurs with 2.59% radioactive lutetium-176
- Rhenium-185* naturally occurs with majority (62.6%) radioactive isotope rhenium-187
- Gold-197
See also
References
- ↑ N. E. Holden, "Standard Atomic Weight Values for the Mononuclidic Elements - 2001," BNL-NCS-68362, Brookhaven National Laboratory (2001)
- ↑ Until 2003, 209Bi was thought to be in the first category. It was then found to have a half-life of 1019 years, about a billion times the age of the universe. See Bismuth