Morphinone reductase is an enzyme which catalyzes the NADH-dependent saturation of the carbon-carbon double bond of morphinone and codeinone, yielding hydromorphone and hydrocodone respectively.[1] This saturation reaction is assisted by a FMN cofactor and the enzyme is a member of the α/β-barrel flavoprotein family.[1] The sequence of the enzyme has been obtained from bacteria Pseudomonas putida M10[2] and has been successfully expressed in yeast[3] and other bacterial species.[4] The enzyme is reported to harbor high sequence and structural similarity to the Old Yellow Enzyme, a large group of flavin-dependent redox biocatalysts of yeast species,[5] and an oestrogen-binding protein of Candida albicans.[4] The enzyme has demonstrated value in biosynthesis of semi-opiate drugs in microorganisms,[6] expanding the chemical diversity of BIA biosynthesis.[7][8]
Enzyme structure
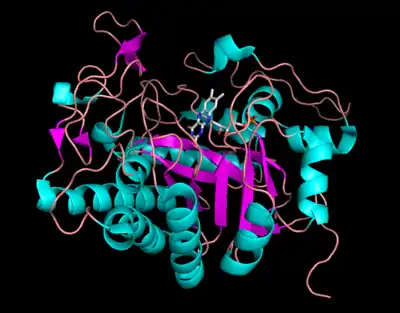
Morphinone reductase is a dimeric flavoenzyme comprising two 8-fold α/β-barrel domains, each with a non-covalently bound FMN prosthetic group located toward the center and C-terminal end of the barrel.[9] At the active site, the Cys-191 residue serves as a proton donor in the oxidative half-reaction with a/ß unsaturated enones. Residues His-186 and Asn-189 are involved in ligand binding and they represent a conserved feature which is also observed in Old Yellow Enzymes.[10] The residue Arg-238 contributes to a key interaction with the flavin as the side chain of this group is located close to the N-1/C-2 carbonyl region of the flavin isoalloxazine ring, stabilizing the negative charge developed during enzyme reduction.
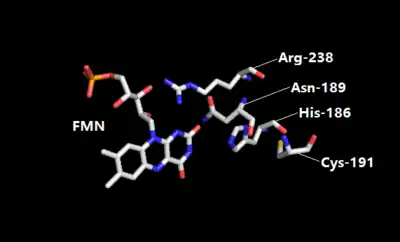 FMN prosthetic group and key residues at the active site of morphinone reductase.[11] |
Enzyme catalysis
Morphinone reductase takes two substrates, namely morphinone and codeinone, and converts them to hydromorphone and hydrocodone respectively. The catalysis is part of the degradation pathway of morphine and codeine in Pseudomonas putida M10: morphine dehydrogenase and morphinone reductase together form a two-step catalysis converting morphine to hydromorphone, and codeine to hydrocondone.[1]
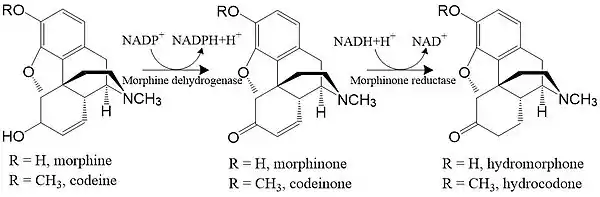 Initial steps of the morphine degradation pathway in Pseudomonas putida M10.[1] |
 A proposed reduction mechanism catalyzed by morphinone reductase.[12] |
The prosthetic group FMN serves as a cofactor in the redox reaction catalyzed by morphinone reductase.[1] In the reduction of morphinone to hydromorphone, FMNH2 is oxidized to FMN which is then reduced by NADH (and H+) to regenerate FMNH2. Previous studies[1][9] showed that NADPH could not be used as a reducing agent, which suggested the enzyme's specific toward NADH as a substrate. Studies have shown that the mechanism of flavin reduction in morphinone reductase involve the rapid formation of an E-NADHCT charge-transfer intermediate prior to FMN reduction.[12][13] It was suggested that the enzyme adapts a two-step kinetic model where the oxidized enzyme (state A) undergoes an enzyme-coenzyme charge-transfer intermediate (state B) before regenerating the reduced form of FMN cofactor (state C). The reduction mechanism involves transfer of a hydride ion from the N5 atom of FMN to 2-cyclohexenone. The other proton donor for the saturation reaction remained uncertain.[9]
Steady-state kinetics experiments suggested that morphinone reductase may follow a hybrid two-site, ping-pong kinetic mechanism[1] in which the alkaloid substrates, i.e. morphinone and codeinone, bind independently at separate sites and sequential redox reaction is facilitated by reducing equivalents passed between the binding sites by means of redox-active prosthetic groups, i.e. FMN.
Potential regulatory mechanism
The enzyme was shown to be highly similar to an oestrogen-binding protein from the fungus species Candida albicans,[1][14] in which the binding of oestrogen prevents the reduction of 2-cyclohexenone. In addition, previous experimental characterization results[1] suggested that morphinone reductase activity is competitively inhibited by progesterone and cortisone, which indicates that the regulatory mechanism of morphinone reductase could be related to its structural resemblance to the oestrogen-binding protein.
Industrial relevance
The enzyme yields hydromorphone and hydrocodone, which are both valuable semi-synthetic opiate drugs. Hydromorphone is a powerful analgesic, which is shown to be more potent than morphine.[15] Hydrocodone is a FDA-approved, mild analgesic and antitussive.[16]
Notably, morphine and codeine are natural products of the opiate biosynthetic pathway in opium poppy plant Papaver somniferum. Modern technologies in genetic engineering and metabolic engineering enabled the production of these natural products in microorganisms.[17] Complete biosynthesis of opiate compounds has been achieved in genetically tractable organisms Saccharomyces cerevisiae[3] and Escherichia coli.[18] Morphinone reductase was also successfully expressed in these two organisms.[3][4][19] The enzyme represents a promising candidate for downstream modifications of opiate compounds, allowing the biosynthesis of valuable semi-synthetic opiate drugs in microorganisms.[6] As an example, morphinone reductase was used as part of the de novo biosynthetic pathway of hydrocodone in yeast.[3]
References
- 1 2 3 4 5 6 7 8 9 French, C E; Bruce, N C (1 July 1994). "Purification and characterization of morphinone reductase from Pseudomonas putida M10". Biochemical Journal. 301 (Pt 1): 97–103. doi:10.1042/bj3010097. ISSN 0264-6021. PMC 1137148. PMID 8037698.
- ↑ "morB - Morphinone reductase - Pseudomonas putida - morB gene & protein". www.uniprot.org.
- 1 2 3 4 Galanie, Stephanie; Thodey, Kate; Trenchard, Isis J.; Interrante, Maria Filsinger; Smolke, Christina D. (4 September 2015). "Complete biosynthesis of opioids in yeast". Science. 349 (6252): 1095–1100. doi:10.1126/science.aac9373. ISSN 0036-8075. PMC 4924617. PMID 26272907.
- 1 2 3 French, C. E.; Bruce, N. C. (15 December 1995). "Bacterial morphinone reductase is related to Old Yellow Enzyme". The Biochemical Journal. 312 ( Pt 3) (3): 671–678. doi:10.1042/bj3120671. ISSN 0264-6021. PMC 1136166. PMID 8554504.
- ↑ Toogood, Helen S.; Gardiner, John M.; Scrutton, Nigel S. (9 August 2010). "Biocatalytic Reductions and Chemical Versatility of the Old Yellow Enzyme Family of Flavoprotein Oxidoreductases". ChemCatChem. 2 (8): 892–914. doi:10.1002/cctc.201000094. ISSN 1867-3899. S2CID 97812184.
- 1 2 Thodey, Kate; Galanie, Stephanie; Smolke, Christina D. (1 October 2014). "A microbial biomanufacturing platform for natural and semisynthetic opioids". Nature Chemical Biology. 10 (10): 837–844. doi:10.1038/nchembio.1613. ISSN 1552-4450. PMC 4167936. PMID 25151135.
- ↑ Minami, Hiromichi; Kim, Ju-Sung; Ikezawa, Nobuhiro; Takemura, Tomoya; Katayama, Takane; Kumagai, Hidehiko; Sato, Fumihiko (27 May 2008). "Microbial production of plant benzylisoquinoline alkaloids". Proceedings of the National Academy of Sciences. 105 (21): 7393–7398. doi:10.1073/pnas.0802981105. ISSN 0027-8424. PMC 2396723. PMID 18492807.
- ↑ Ziegler, Jörg; Facchini, Peter J. (1 January 2008). "Alkaloid biosynthesis: metabolism and trafficking". Annual Review of Plant Biology. 59: 735–769. doi:10.1146/annurev.arplant.59.032607.092730. ISSN 1543-5008. PMID 18251710. S2CID 40585078.
- 1 2 3 4 Barna, Terez; Messiha, Hanan Latif; Petosa, Carlo; Bruce, Neil C.; Scrutton, N.S.; Moody, P.C. (23 August 2002). "Crystal structure of bacterial morphinone reductase and properties of the C191A mutant enzyme". The Journal of Biological Chemistry. 277 (34): 30976–30983. doi:10.1074/jbc.M202846200. hdl:2437/124229. ISSN 0021-9258. PMID 12048188.
- ↑ Brown, B. J.; Deng, Z.; Karplus, P. A.; Massey, V. (4 December 1998). "On the active site of Old Yellow Enzyme. Role of histidine 191 and asparagine 194". The Journal of Biological Chemistry. 273 (49): 32753–32762. doi:10.1074/jbc.273.49.32753. ISSN 0021-9258. PMID 9830019.
- ↑ Messiha, Hanan L.; Bruce, Neil C.; Sattelle, Benedict M.; Sutcliffe, Michael J.; Munro, Andrew W.; Scrutton, Nigel S. (22 July 2005). "Role of Active Site Residues and Solvent in Proton Transfer and the Modulation of Flavin Reduction Potential in Bacterial Morphinone Reductase". Journal of Biological Chemistry. 280 (29): 27103–27110. doi:10.1074/jbc.M502293200. ISSN 0021-9258. PMID 15905167.
- 1 2 Basran, Jaswir; Harris, Richard J.; Sutcliffe, Michael J.; Scrutton, Nigel S. (7 November 2003). "H-tunneling in the Multiple H-transfers of the Catalytic Cycle of Morphinone Reductase and in the Reductive Half-reaction of the Homologous Pentaerythritol Tetranitrate Reductase". Journal of Biological Chemistry. 278 (45): 43973–43982. doi:10.1074/jbc.M305983200. ISSN 0021-9258. PMID 12941965.
- ↑ Craig, Daniel H.; Moody, Peter C. E.; Bruce, Neil C.; Scrutton, Nigel S. (1 May 1998). "Reductive and Oxidative Half-Reactions of Morphinone Reductase from Pseudomonas putida M10: A Kinetic and Thermodynamic Analysis". Biochemistry. 37 (20): 7598–7607. doi:10.1021/bi980345i. ISSN 0006-2960. PMID 9585575.
- ↑ Madani, N D; Malloy, P J; Rodriguez-Pombo, P; Krishnan, A V; Feldman, D (1 February 1994). "Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol". Proceedings of the National Academy of Sciences of the United States of America. 91 (3): 922–926. doi:10.1073/pnas.91.3.922. ISSN 0027-8424. PMC 521425. PMID 8302868.
- ↑ Sarhill, N.; Walsh, D.; Nelson, K. A. (1 March 2001). "Hydromorphone: pharmacology and clinical applications in cancer patients". Supportive Care in Cancer. 9 (2): 84–96. doi:10.1007/s005200000183. ISSN 0941-4355. PMID 11305075. S2CID 22849970.
- ↑ "Hydrocodone: Uses, Side Effects & Dosage Guide - Drugs.com". Drugs.com.
- ↑ Smanski, Michael J.; Zhou, Hui; Claesen, Jan; Shen, Ben; Fischbach, Michael A.; Voigt, Christopher A. (1 March 2016). "Synthetic biology to access and expand nature's chemical diversity". Nature Reviews. Microbiology. 14 (3): 135–149. doi:10.1038/nrmicro.2015.24. ISSN 1740-1534. PMC 5048682. PMID 26876034.
- ↑ Nakagawa, A; Matsumura, E; Koyanagi, T; Katayama, T; Kawano, N; Yoshimatsu, K; Yamamoto, K; Kumagai, H; Sato, F; Minami, H (5 February 2016). "Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli". Nature Communications. 7: 10390. doi:10.1038/ncomms10390. ISSN 2041-1723. PMC 4748248. PMID 26847395.
- ↑ French, C. E.; Hailes, A. M.; Rathbone, D. A.; Long, M. T.; Willey, D. L.; Bruce, N. C. (1 July 1995). "Biological production of semisynthetic opiates using genetically engineered bacteria". Nature Biotechnology. 13 (7): 674–676. doi:10.1038/nbt0795-674. ISSN 0733-222X. PMID 9634804. S2CID 20602139.